Pirfenidone
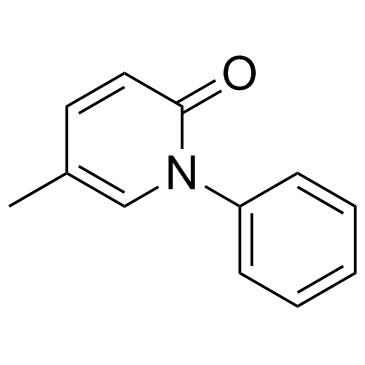
Pirfenidone structure
|
Common Name | Pirfenidone | ||
|---|---|---|---|---|
| CAS Number | 53179-13-8 | Molecular Weight | 185.222 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 329.1±15.0 °C at 760 mmHg | |
| Molecular Formula | C12H11NO | Melting Point | 96-97ºC | |
| MSDS | Chinese USA | Flash Point | 152.7±11.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of PirfenidonePirfenidone is a drug used for the treatment of idiopathic pulmonary fibrosis. It inhibits FGFR, EGFR, PDGFR, TGF-β, thereby slowing tumor cell proliferation. |
| Name | pirfenidone |
|---|---|
| Synonym | More Synonyms |
| Description | Pirfenidone is a drug used for the treatment of idiopathic pulmonary fibrosis. It inhibits FGFR, EGFR, PDGFR, TGF-β, thereby slowing tumor cell proliferation. |
|---|---|
| Related Catalog | |
| Target |
TGF-β2[1] |
| In Vitro | Pirfenidone (PFD) reduces the protein levels of the matrix metalloproteinase (MMP)-11, a TGF-β target gene and furin substrate involved in carcinogenesis. These data define PFD or PFD-related agents as promising agents for human cancers associated with enhanced TGF-β activity[1]. In RAW264.7 cells, a murine macrophage-like cell line, Pirfenidone suppresses the proinflammatory cytokine TNF-α by a translational mechanism, which is independent of activation of the MAPK2, p38 MAPK, and JNK. In the murine endotoxin shock model, Pirfenidone potently inhibits the production of the proinflammatory cytokines, TNF-α, interferon-γ, and interleukin-6, but enhances the production of the anti-inflammatory cytokine, interleukin-10[2]. Pirfenidone (PFD) shows its inhibitory effects on the proliferation of HLECs. Cell proliferation is attenuated in the 0.3 mg/mL group after 24 hours compare with the control group (P=0.044). The effect is more apparent in the 0.5 mg/mL group at 24, 48, and 72 hours (P<0.05). The proliferation is almost completely inhibited with 1 mg/mL PFD at all the time-points (P<0.01)[3]. |
| In Vivo | Administration of Pirfenidone (300 mg/kg/day) for 4 wk. Pirfenidone significantly attenuates the score when administered in Bleomycin (BLM)-treated mice (P<0.0001). Moreover, collagen content is quantified in the lungs to evaluate the anti-fibrotic effects of Pirfenidone. The collagen content in the lungs of BLM-treated mice is significantly increased compared with that in saline- or Pirfenidone-treated mice, and this increase is significantly attenuated by Pirfenidone administration on day 28 after BLM treatment (P=0.0012)[4]. |
| Cell Assay | HLECs are seeded in 96-well plates (1×104 cells/well) for 24 hours in α-MEM/10% FBS/1%NEAA, and are cultured in stationary tubes in serum-free medium for 24 hours. And then the culture medium is removed and cells are bathed in α-MEM with 10% FBS and 1% NEAA supplemented with 0, 0.01, 0.1, 0.2, 0.3, 0.5, or 1 mg/mL Pirfenidone for 0, 4, 12, 24, 48, or 72 hours. After incubation with 180 µL α-MEM and 20 µL of 5 mg/mL MTT for 4 hours at 37°C, the MTT solution is discarded. The Formosan precipitates are dissolved in 180 µL DMSO by agitating the dishes for 10 minutes at 200 rpm on an orbital shaker. The absorbance at 490 nm in each well is read with a micro plate reader. We further examined the effects of PFD by refining the concentrations at 0.2, 0.25, 0.3, 0.4, 0.5 and 0.6 mg/mL using the MTT assay[3]. |
| Animal Admin | Mice[4] Nine-week-old female C57BL/6 mice are used. Pirfenidone is administered orally for 14 days after osmotic pump implantation. The volume of administration is determined according to body weight. Animals are allocated into 4 groups (n=6/group): normal control, BLM, Pirfenidone (300 mg/kg/day), and BLM + Pirfenidone. The Pirfenidone dose is selected according to a report published elsewhere. Pirfenidone is also administered in a therapeutic setting beginning at day 10 to assess the effect of the drug on the fibrotic phase of BLM model mice. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 329.1±15.0 °C at 760 mmHg |
| Melting Point | 96-97ºC |
| Molecular Formula | C12H11NO |
| Molecular Weight | 185.222 |
| Flash Point | 152.7±11.6 °C |
| Exact Mass | 185.084061 |
| PSA | 22.00000 |
| LogP | 1.82 |
| Appearance of Characters | solid |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.592 |
| Storage condition | Store at RT |
| Water Solubility | DMSO: ≥10 mg/mL, soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UV1148200 |
| HS Code | 2933399090 |
|
~56% 
Pirfenidone CAS#:53179-13-8 |
| Literature: AUSPEX PHARMACEUTICALS, INC.; ZHANG, Chengzhi; SOMMERS, Andreas Patent: WO2012/122165 A2, 2012 ; Location in patent: Page/Page column 62 ; |
|
~% 
Pirfenidone CAS#:53179-13-8 |
| Literature: WO2010/141600 A2, ; Page/Page column 13-14 ; |
|
~% 
Pirfenidone CAS#:53179-13-8 |
| Literature: US2008/161361 A1, ; Page/Page column 8-9 ; |
|
~98% 
Pirfenidone CAS#:53179-13-8 |
| Literature: Crifar, Cynthia; Petiot, Pauline; Ahmad, Tabinda; Gagnon, Alexandre Chemistry - A European Journal, 2014 , vol. 20, # 10 p. 2755 - 2760 |
|
~% 
Pirfenidone CAS#:53179-13-8 |
| Literature: US3974281 A1, ; US 3974281 A |
|
~11% 
Pirfenidone CAS#:53179-13-8 |
| Literature: Kader, Kamelia F. Abd El; Bialy, Serry A.A. El; El-Ashmawy, Mahmoud B.; Boykin, David W. Heterocyclic Communications, 2012 , vol. 18, # 4 p. 193 - 197 |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Lung fibrosis: drug screening and disease biomarker identification with a lung slice culture model and subtracted cDNA Library.
Altern. Lab. Anim. 42(4) , 235-43, (2014) Pulmonary fibrosis is a progressive and irreversible disorder with no appropriate cure. A practical and effective experimental model that recapitulates the disease will greatly benefit the research co... |
|
|
A novel genomic signature with translational significance for human idiopathic pulmonary fibrosis.
Am. J. Respir. Cell. Mol. Biol. 52(2) , 217-31, (2015) The bleomycin-induced rodent lung fibrosis model is commonly used to study mechanisms of lung fibrosis and to test potential therapeutic interventions, despite the well recognized dissimilarities to h... |
|
|
Local delivery of biodegradable pirfenidone nanoparticles ameliorates bleomycin-induced pulmonary fibrosis in mice.
Nanotechnology 23(50) , 505101, (2012) Our purpose was to assess sustained delivery and enhanced efficacy of pirfenidone-loaded nanoparticles after intratracheal instillation.Poly(lactide-co-glycolide) nanoparticles containing pirfenidone ... |
| TORASEMIDETECHPIRFENIDONE |
| 5-Methyl-1-phenyl-2-(1H)-pyridone |
| 2(1H)-Pyridinone, 5-methyl-1-phenyl- |
| 5-methyl-1-phenyl-2-pyridone |
| 5-Methyl-N-phenyl-2-1H-pyridone |
| Pirespa |
| S-7701 |
| amr-69 |
| 5-methyl-1-phenyl-1H-pyridin-2-one |
| 5-Methyl-1-phenyl-2(1H)-pyridinone |
| 5-methyl-1-phenyl-pyridin-2-one |
| Etuary |
| 5-Methyl-1-phenylpyridin-2(1H)-one |
| MFCD00866047 |
| Pirfenidone |
| 5-21-07-00197 (Beilstein Handbook Reference) |
| Esbriet |