methocarbamol
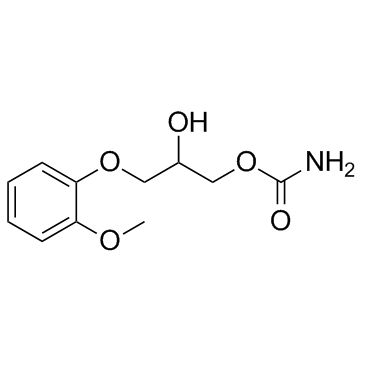
methocarbamol structure
|
Common Name | methocarbamol | ||
|---|---|---|---|---|
| CAS Number | 532-03-6 | Molecular Weight | 241.240 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 472.5±40.0 °C at 760 mmHg | |
| Molecular Formula | C11H15NO5 | Melting Point | 95-97ºC | |
| MSDS | Chinese USA | Flash Point | 239.6±27.3 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of methocarbamolMethocarbamol is a central muscle relaxant used to treat skeletal muscle spasms.Target: Carbonic AnhydraseMethocarbamol is the carbamate of guaifenesin, but does not produce guaifenesin as a metabolite, because the carbamate bond is not hydrolyzed metabolically; metabolism is by Phase I ring hydroxylation and O-demethylation, followed by Phase II conjugation. All the major metabolites are unhydrolyzed carbamates. Methocarbamol is used as an adjunct in the symptomatic treatment of musculoskeletal conditions associated with painful muscle spasm [1, 2]. |
| Name | Methocarbamol |
|---|---|
| Synonym | More Synonyms |
| Description | Methocarbamol is a central muscle relaxant used to treat skeletal muscle spasms.Target: Carbonic AnhydraseMethocarbamol is the carbamate of guaifenesin, but does not produce guaifenesin as a metabolite, because the carbamate bond is not hydrolyzed metabolically; metabolism is by Phase I ring hydroxylation and O-demethylation, followed by Phase II conjugation. All the major metabolites are unhydrolyzed carbamates. Methocarbamol is used as an adjunct in the symptomatic treatment of musculoskeletal conditions associated with painful muscle spasm [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 472.5±40.0 °C at 760 mmHg |
| Melting Point | 95-97ºC |
| Molecular Formula | C11H15NO5 |
| Molecular Weight | 241.240 |
| Flash Point | 239.6±27.3 °C |
| Exact Mass | 241.095016 |
| PSA | 91.01000 |
| LogP | 0.55 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.541 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H317-H334 |
| Precautionary Statements | P261-P280-P284-P304 + P340-P333 + P313-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R42/43 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TY8750000 |
| HS Code | 2924299090 |
|
~% 
methocarbamol CAS#:532-03-6 |
| Literature: Monatshefte fuer Chemie, , vol. 94, p. 339 - 358 |
|
~% 
methocarbamol CAS#:532-03-6 |
| Literature: Monatshefte fuer Chemie, , vol. 94, p. 339 - 358 |
|
~% 
methocarbamol CAS#:532-03-6 |
| Literature: Monatshefte fuer Chemie, , vol. 94, p. 339 - 358 |
|
~% 
methocarbamol CAS#:532-03-6 |
| Literature: Monatshefte fuer Chemie, , vol. 94, p. 339 - 358 |
|
~% 
methocarbamol CAS#:532-03-6 |
| Literature: Monatshefte fuer Chemie, , vol. 94, p. 339 - 358 |
|
~% 
methocarbamol CAS#:532-03-6 |
| Literature: Monatshefte fuer Chemie, , vol. 94, p. 339 - 358 |
|
~% 
methocarbamol CAS#:532-03-6 |
| Literature: Yakugaku Zasshi, , vol. 76, p. 880 Chem.Abstr., , p. 2625 US2770649 , ; DE1018412 , ; |
|
~% 
methocarbamol CAS#:532-03-6 |
| Literature: Monatshefte fuer Chemie, , vol. 94, p. 339 - 358 |
|
~% 
methocarbamol CAS#:532-03-6
Detail
|
| Literature: Journal of Organic Chemistry, , vol. 22, p. 1595,1598 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
A new HPLC technique for the separation of methocarbamol enantiomers.
J. Pharm. Pharmacol. 51(7) , 873-5, (1999) We have developed a stereoselective high-performance liquid chromatography technique for analytical separation of methocarbamol enantiomers. Precolumn derivatization was performed at room temperature ... |
|
|
Spectrofluorometric determination of methocarbamol in pharmaceutical preparations and human plasma.
J. Fluoresc. 21(2) , 555-61, (2011) A simple, sensitive and rapid spectrofluorometric method for determination of methocarbamol in pharmaceutical formulations and spiked human plasma has been developed. The proposed method is based on t... |
|
|
A stability-indicating high-performance liquid chromatographic method for the determination of methocarbamol in veterinary preparations.
J. AOAC Int. 92(5) , 1602-5, (2009) An isocratic HPLC method was developed and validated for the quantitation of methocarbamol in the presence of its degradation products. Quantitation was achieved using a reversed-phase C18 column at a... |
| Guaiacol glyceryl ether carbamate |
| neuraxin |
| Relax |
| Methyocarbamol |
| Delaxin |
| 2-hydroxy-3-{[2-(methyloxy)phenyl]oxy}propyl carbamate |
| Relestrid |
| Carbamic Acid 2-Hydroxy-3-(2-methoxyphenoxy)propyl Ester |
| guaifenesin carbamate |
| EINECS 208-524-3 |
| 2-Hydroxy-3-(2-methoxyphenoxy)propyl carbamate |
| Lumirelax |
| Miolaxene |
| Robaxan |
| Perilax |
| MFCD00057662 |
| methocarbamol |
| Robamol |
| AHR 85 |
| Traumacut |
| Miowas |
| Robaxin |
| 1,2-Propanediol, 3-(2-methoxyphenoxy)-, 1-carbamate |
| Avetil |


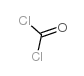
![[1-Chloro-3-(2-methoxyphenoxy)propan-2-yl] carbamate structure](https://image.chemsrc.com/caspic/478/2049-22-1.png)

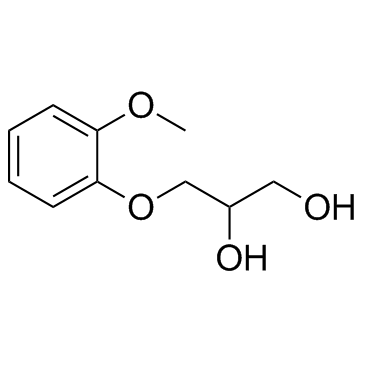 CAS#:93-14-1
CAS#:93-14-1
