Tropinone
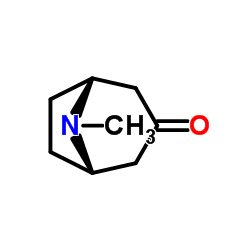
Tropinone structure
|
Common Name | Tropinone | ||
|---|---|---|---|---|
| CAS Number | 532-24-1 | Molecular Weight | 139.195 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 217.1±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H13NO | Melting Point | 40-44 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 90.0±0.0 °C | |
Use of TropinoneTropinone, an alkaloid, acts as a synthetic intermediate to Atropine[1]. |
| Name | tropinone |
|---|---|
| Synonym | More Synonyms |
| Description | Tropinone, an alkaloid, acts as a synthetic intermediate to Atropine[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 217.1±0.0 °C at 760 mmHg |
| Melting Point | 40-44 °C(lit.) |
| Molecular Formula | C8H13NO |
| Molecular Weight | 139.195 |
| Flash Point | 90.0±0.0 °C |
| Exact Mass | 139.099716 |
| PSA | 20.31000 |
| LogP | 0.07 |
| Vapour Pressure | 0.1±0.4 mmHg at 25°C |
| Index of Refraction | 1.505 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S23-S24/25-S36/37/39-S26-S22 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Toxicity of field bindweed (Convolvulus arvensis) to mice.
Vet. Hum. Toxicol. 37(5) , 452-4, (1995) The effects of feeding high and low doses of field bindweed (Convolvulus arvensis) to mice were investigated. Bindweed contains several alkaloids, including pseudotropine, and lesser amounts of tropin... |
|
|
Synthesis and applications of masked oxo-sulfinamides in asymmetric synthesis.
Org. Biomol. Chem. 10(26) , 5021-31, (2012) This short perspective reports on the synthesis and applications of a class of chiral amino carbonyl compounds, masked oxo-sulfinamides where the amine is protected with an N-sulfinyl moiety and the c... |
|
|
Biosynthesis of calystegines: 15N NMR and kinetics of formation in root cultures of Calystegia sepium.
Phytochemistry 62(3) , 325-32, (2003) Calystegines are nortropane alkaloids bearing between three and five hydroxyl groups in various positions. [15N]Tropinone was administered to root cultures of Calystegia sepium and the incorporation i... |
| Tropanon |
| N-methyl-3-tropanone |
| 8-azabicyclo[3.2.1]octan-3-one, 8-methyl-, (1S,5S)- |
| 8-Methyl-8-azabicyclo[3.2.1]-3-octanone |
| 3-tropinone |
| tropan-3-one |
| (1S,5S)-8-Methyl-8-azabicyclo[3.2.1]octan-3-one |
| Tropinone |
| EINECS 208-530-6 |
| Tropinon |
| Tropanone |
| 3-Tropanone |
| 8-Azabicyclo[3.2.1]octan-3-one, 8-methyl-, (1R,5S)- |
| (1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-one |
| 1αH,5αH-tropan-3-one |
| 8-methyl-8-aza-bicyclo[3.2.1]octan-3-one |
| MFCD00005549 |
| 1αH,5αH-Tropan-3-one (8CI) |
| TROPIONONE |
| 8-methyl-8-azabicyclo[3.2.1]oct-3-one |
| 8-methyl-8-azabicyclo[3.2.1]octane-3-one |
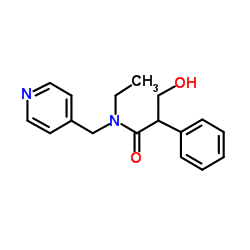 CAS#:120-29-6
CAS#:120-29-6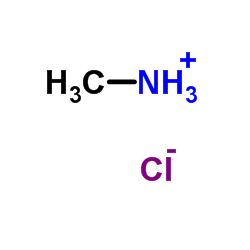 CAS#:593-51-1
CAS#:593-51-1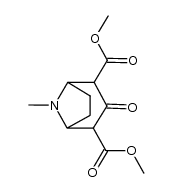 CAS#:100371-46-8
CAS#:100371-46-8 CAS#:502-41-0
CAS#:502-41-0 CAS#:502-42-1
CAS#:502-42-1 CAS#:1121-66-0
CAS#:1121-66-0 CAS#:135-97-7
CAS#:135-97-7![2β-bromo-8-methyl-8-azabicyclo[3.2.1]octan-3-one Structure](https://image.chemsrc.com/caspic/493/62251-43-8.png) CAS#:62251-43-8
CAS#:62251-43-8 CAS#:5690-89-1
CAS#:5690-89-1![Ethyl 3-oxo-8-azabicyclo[3.2.1]octane-8-carboxylate structure](https://image.chemsrc.com/caspic/055/32499-64-2.png) CAS#:32499-64-2
CAS#:32499-64-2![8-azabicyclo[3.2.1]octan-3-one structure](https://image.chemsrc.com/caspic/036/5632-84-8.png) CAS#:5632-84-8
CAS#:5632-84-8 CAS#:36127-17-0
CAS#:36127-17-0![8-methyl-3-(2-methylphenyl)-8-azabicyclo[3.2.1]oct-3-ene structure](https://image.chemsrc.com/caspic/277/185099-65-4.png) CAS#:185099-65-4
CAS#:185099-65-4 CAS#:22932-26-9
CAS#:22932-26-9![ethyl 2-(8-methyl-8-azabicyclo[3.2.1]octan-3-ylidene)acetate structure](https://image.chemsrc.com/caspic/066/2858-77-7.png) CAS#:2858-77-7
CAS#:2858-77-7![ethyl 2-(8-methyl-8-azabicyclo[3.2.1]oct-3-en-3-yl)acetate structure](https://image.chemsrc.com/caspic/173/65180-06-5.png) CAS#:65180-06-5
CAS#:65180-06-5![8-BOC-3-(TRIFLUOROMETHYLSULFONYLOXY)-8-AZABICYCLO[3.2.1]OCT-3-ENE structure](https://image.chemsrc.com/caspic/163/185099-68-7.png) CAS#:185099-68-7
CAS#:185099-68-7 CAS#:185099-67-6
CAS#:185099-67-6
