pterostilbene
pterostilbene structure
|
Common Name | pterostilbene | ||
|---|---|---|---|---|
| CAS Number | 537-42-8 | Molecular Weight | 256.296 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 420.5±35.0 °C at 760 mmHg | |
| Molecular Formula | C16H16O3 | Melting Point | 89-92ºC | |
| MSDS | Chinese USA | Flash Point | 208.1±25.9 °C | |
| Symbol |


GHS05, GHS09 |
Signal Word | Danger | |
Use of pterostilbenePterostilbene is a stilbenoid isolated from blueberries and Pterocarpus marsupium[1]. Shows anti-oxidant, anti-inflammatory, anti-carcinogenic, anti-diabetic and anti-obesity properties[1][4]. Pterostilbene blocks ROS production[3], also exhibits inhibitory activity against various free radicals such as DPPH, ABTS, hydroxyl, superoxide and hydrogen peroxide[4]. |
| Name | pterostilbene |
|---|---|
| Synonym | More Synonyms |
| Description | Pterostilbene is a stilbenoid isolated from blueberries and Pterocarpus marsupium[1]. Shows anti-oxidant, anti-inflammatory, anti-carcinogenic, anti-diabetic and anti-obesity properties[1][4]. Pterostilbene blocks ROS production[3], also exhibits inhibitory activity against various free radicals such as DPPH, ABTS, hydroxyl, superoxide and hydrogen peroxide[4]. |
|---|---|
| Related Catalog | |
| In Vitro | Pterostilbene (0, 5, 25, 50, 100, 200 and 400 µM) shows inhibitory activity against the growth of HeLa cells, with IC50s of 101.2 µM and 65.9 µM at 24 and 48 hrs, respectively. Ipterostilbene (0, 25, 100 and 200 µM) also induces the apoptosis HeLa cells[2]. Pterostilbene (0.05, 0.1, 0.15 and 0.2 mM) has high anti-oxidant activity against DPPH, ABTS, hydroxyl, superoxide, hydrogen peroxide in a dose-dependent manner. Pterostilbene decreases lipid peroxides and hydroperoxides, reduces protein carbonyl groups and restores protein sulphydryl groups in response to damage by TBHP and As-Fe2+. Pterostilbene also inhibits single strand breaks in pBR322[4]. |
| In Vivo | Pterostilbene (30 mg/kg daily, p.o. for 21 days) inhibits reactive oxygen species production in the animal model of inflammation[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 420.5±35.0 °C at 760 mmHg |
| Melting Point | 89-92ºC |
| Molecular Formula | C16H16O3 |
| Molecular Weight | 256.296 |
| Flash Point | 208.1±25.9 °C |
| Exact Mass | 256.109955 |
| PSA | 38.69000 |
| LogP | 4.13 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.640 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: >20mg/mL |
| Symbol |


GHS05, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H318-H411 |
| Precautionary Statements | P273-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R41;R51/53 |
| Safety Phrases | S26-S39-S61 |
| RIDADR | UN 3077 |
| Packaging Group | Ⅲ |
| Hazard Class | 9.0 |
| HS Code | 2909500000 |
| Precursor 7 | |
|---|---|
| DownStream 4 | |
| HS Code | 2909500000 |
|---|---|
| Summary | 2909500000 ether-phenols, ether-alcohol-phenols and their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377.
Free Radic. Biol. Med. 83 , 214-26, (2015) High dietary fructose is an important causative factor in the development of metabolic syndrome-associated glomerular podocyte oxidative stress and injury. Here, we identified microRNA-377 (miR-377) a... |
|
|
Anti-proliferative effect of pterostilbene on rat hepatoma cells in culture.
Cytotechnology 67 , 671-80, (2015) Pterostilbene, a methoxylated analogue of resveratrol, is a natural compound primarily found in blueberries and several types of grapes. However, little is known about the effect of pterostilbene on t... |
|
|
Involvement of the Nrf2 pathway in the regulation of pterostilbene-induced apoptosis in HeLa cells via ER stress.
J. Pharmacol. Sci. 126(3) , 216-29, (2014) Among the various cancer cell lines, HeLa cells were found to be sensitive to pterostilbene (Pte), a compound that is enriched in small fruits such as grapes and berries. However, the mechanism involv... |
| 4-(3,5-Dimethoxystyryl)phenol |
| 4-[(E)-2-(3,5-Diméthoxyphényl)éthènyl]phénol |
| Phenol, 4-(2-(3,5-dimethoxyphenyl)ethenyl)-, (E)- |
| 3,5-Dimethoxy-4'-hydroxy-trans-stilbene |
| 3',5'-Dimethoxy-4-stilbenol |
| 4-(2-(3,5-Dimethoxyphenyl)ethenyl)phenol |
| 4-[(E)-2-(3,5-Dimethoxyphenyl)vinyl]phenol |
| (E)-4-(3,5-Dimethoxystyryl)phenol |
| trans-1-(3,5-Dimethoxyphenyl)-2-(4-hydroxyphenyl)ethylene |
| Pterostilbene |
| 4-[(E)-2-(3,5-dimethoxyphenyl)ethenyl]phenol |
| Phenol, 4-[(E)-2-(3,5-dimethoxyphenyl)ethenyl]- |
| Pterostilbene,Pterocarpus marsupium |
| (E)-pterostilbene |
| 4-Stilbenol, 3',5'-dimethoxy-, (E)- |
| 3,5-dimethoxy-4'-hydroxystilbene |
| 4'-hydroxy-3,5-dimethoxystilbene |
| 4'-Hydroxy-3,5-dimethoxy-trans-stilbene |
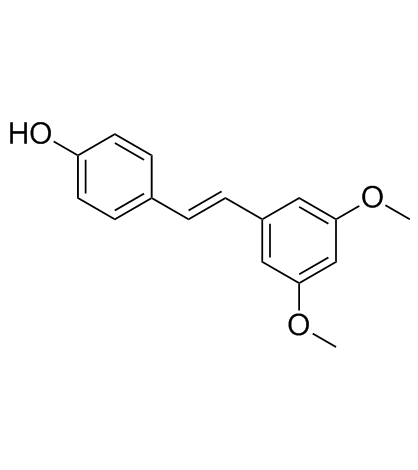
 CAS#:25245-27-6
CAS#:25245-27-6 CAS#:2628-17-3
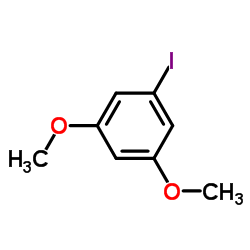
CAS#:2628-17-3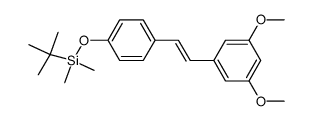 CAS#:441351-31-1
CAS#:441351-31-1 CAS#:7311-34-4
CAS#:7311-34-4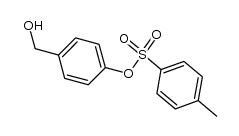 CAS#:109103-16-4
CAS#:109103-16-4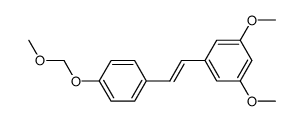 CAS#:848487-76-3
CAS#:848487-76-3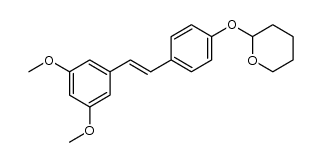 CAS#:1257314-98-9
CAS#:1257314-98-9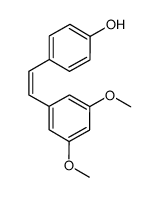 CAS#:441351-32-2
CAS#:441351-32-2 CAS#:42438-89-1
CAS#:42438-89-1 CAS#:1132-21-4
CAS#:1132-21-4![4-[2-(3,5-dimethoxyphenyl)ethyl]phenol structure](https://image.chemsrc.com/caspic/043/116518-94-6.png) CAS#:116518-94-6
CAS#:116518-94-6
