| Description |
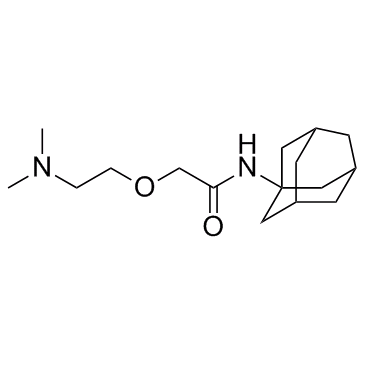
Tromantadine is a herpes simplex virus (HSV) inhibitor.
|
| Related Catalog |
|
| Target |
HSV[1]
|
| In Vitro |
Tromantadine inhibits herpes simplex virus type 1 (KOS strain)-induced cytopathic effect and virus replication with limited toxicity to the cells. Vero and HEp-2 cells tolerate up to 2 mg of Tromantadine per 2x106 cells for 24-, 48-, or 96-h incubation periods with little change in cell morphology. Treatment of the cells with 10 to 50 μg of Tromantadine reduces herpes simplex virus-induced cytopathic effect. Treatment with 100 to 500 μg of Tromantadine inhibits herpes simplex virus-induced cytopathic effect and reduces virus production. Complete inhibition of virus production is observed with treatments of 500 μg to 1 mg. The antiherpetic activity of Tromantadine is dependent upon the viral inoculum size and the time of addition of the compound with respect to infection. Virion synthesis and viral polypeptide synthesis are inhibited by addition of Tromantadine at the time of infection or 4 h postinfection[1]. Tromantadine raises the bilayer to hexagonal phase transition temperature of synthetic phosphatidylethanolamines and is less disruptive to phospholipid packing. Tromantadine acts similar to cyclosporin A, previously demonstrated to inhibit viral-induced cell-cell fusion[2].
|
| Cell Assay |
Confluent monolayers of Vero (2.4x106 per plate) or HEp-2 (1.6x106 per plate) cells are treated with various amounts of Tromantadine 15 min before and then throughout the infection and incubation. The dose of Tromantadine applied, rather than the concentration, is varied since, as for amantadine, the cells concentrate the compound from the medium. For most assays, cells are infected with 6x108 PFU of HSV-1 in 0.5 mL for 1 h at 37°C, and the virus solution is then aspirated off the monolayer and replaced with 2 mL of maintenance medium containing the same amount of the compound. The antiviral activity of the compounds is determined as a reduction in both virally induced cytopathic effect (CPE) and production of extracellular infectious virus 24, 48, and % h postinfection[1].
|
| References |
[1]. Rosenthal KS, et al. Tromantadine: inhibitor of early and late events in herpes simplex virus replication. Antimicrob Agents Chemother. 1982 Dec;22(6):1031-6. [2]. Cheetham JJ, et al. Comparison of the interaction of the anti-viral chemotherapeutic agents amantadine and tromantadine with model phospholipid membranes. Biosci Rep. 1987 Mar;7(3):225-30.
|