12S-HHTrE
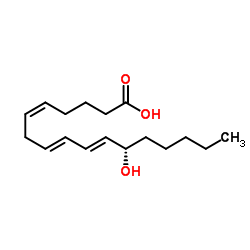
12S-HHTrE structure
|
Common Name | 12S-HHTrE | ||
|---|---|---|---|---|
| CAS Number | 54397-84-1 | Molecular Weight | 280.402 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 451.8±45.0 °C at 760 mmHg | |
| Molecular Formula | C17H28O3 | Melting Point | 151.79 (Mean or Weighted MP)ºC | |
| MSDS | USA | Flash Point | 241.1±25.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 12S-HHTrE12S-HHT (12(S)-HHTrE) is an enzymatic product of prostaglandin H2 (PGH2) derived from cyclooxygenase (COX)-mediated arachidonic acid metabolism. 12S-HHT is an endogenous ligand for BLT2 that fully activates BLT2 in vivo. 12S-HHT suppresses UV-induced IL-6 synthesis in keratinocytes, exerting an anti-inflammatory activity[1][2]. |
| Name | 12(S)-Hydroxy-(5Z,8E,10E)-heptadecatrienoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 12S-HHT (12(S)-HHTrE) is an enzymatic product of prostaglandin H2 (PGH2) derived from cyclooxygenase (COX)-mediated arachidonic acid metabolism. 12S-HHT is an endogenous ligand for BLT2 that fully activates BLT2 in vivo. 12S-HHT suppresses UV-induced IL-6 synthesis in keratinocytes, exerting an anti-inflammatory activity[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | 12S-HHT (0-150 nM; 3 hours) has anti-inflammatory activity by attenuating the UVB-induced IL-6 synthesis in HaCaT cells[2]. 12S-HHT inhibits the UVB-stimulated p38 MAPK/NF-κB pathway by up-regulating MKP-1, which leads to the suppression of IL-6 synthesis[2].12S-HHT is an endogenous agonist for BLT2[3]. Western Blot Analysis[2] Cell Line: HaCaT cells Concentration: 0, 12.5, 25, 75 or 150 nM Incubation Time: 3 hours Result: UVB (5 mJ/cm2) irradiation markedly up-regulated IL-6 synthesis and release, which was suppressed by the treatment with 12-HHT in a concentration-dependent manner. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 451.8±45.0 °C at 760 mmHg |
| Melting Point | 151.79 (Mean or Weighted MP)ºC |
| Molecular Formula | C17H28O3 |
| Molecular Weight | 280.402 |
| Flash Point | 241.1±25.2 °C |
| Exact Mass | 280.203857 |
| PSA | 57.53000 |
| LogP | 4.30 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.507 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | F: Flammable;Xi: Irritant; |
| Risk Phrases | R11 |
| Safety Phrases | 16-26-36 |
| RIDADR | UN 1170 3 |
| HS Code | 2918199090 |
| HS Code | 2918199090 |
|---|---|
| Summary | 2918199090 other carboxylic acids with alcohol function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Perfluorinated carboxylic acids inhibit cyclooxygenase pathway more potently than 12-lipoxygenase pathway in rat platelets.
Prostaglandins Leukot. Essent. Fatty Acids 83(4-6) , 225-8, (2010) Two perfluorinated carboxylic acids (PFCAs), pentadecafluorooctanoic acid (PDFOA) and heptadecafluorononanoic acid (HDFNA), were investigated for potential modulatory effects on the cyclooxygenase (CO... |
|
|
Effects of 3-aminopyridine-induced seizures on platelet eicosanoid synthesis.
Pharmacol. Rep. 60(3) , 345-52, (2008) We investigated the influence of recurrent epileptic seizures on the arachidonic acid (AA) cascade in platelets and brain microvessels, using [(14)C]AA as a tracer substrate and chromatographic determ... |
|
|
The anti-inflammatory activities of an extract and compounds isolated fromPlatycladus orientalis(Linnaeus) Francoin vitroandex vivo
J. Ethnopharmacol. 141(2) , 647-52, (2012) As a Chinese traditional herbal medicine, leaves of Platycladus orientalis (Linnaeus) Franco (LPO) are used to treat coughs, excessive mucus secretion, chronic bronchitis, bronchiectasis, and asthma, ... |
| 5,8,10-Heptadecatrienoic acid, 12-hydroxy-, (5Z,8E,10E,12S)- |
| 12(s)-hht |
| 12S-HHTrE |
| (5Z,8E,10E,12S)-12-Hydroxy-5,8,10-heptadecatrienoic acid |