Columbin
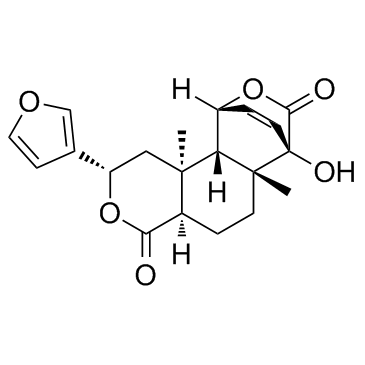
Columbin structure
|
Common Name | Columbin | ||
|---|---|---|---|---|
| CAS Number | 546-97-4 | Molecular Weight | 358.385 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 565.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H22O6 | Melting Point | 190-191ºC | |
| MSDS | USA | Flash Point | 296.0±30.1 °C | |
Use of ColumbinColumbin is a diterpenoid furanolactone with anti-inflammation activity. |
| Name | (1R,2S,3S,5S,8R,11R,12R)-5-(3-Furyl)-12-hydroxy-3,11-dimethyl-6,14-dioxatetracyclo[10.2.2.02,11.03,8]hexadec-15-ene-7,13-dione |
|---|---|
| Synonym | More Synonyms |
| Description | Columbin is a diterpenoid furanolactone with anti-inflammation activity. |
|---|---|
| Related Catalog | |
| In Vitro | Treatment with columbin or l-NAME inhibits LPS/IFN-γ-induced NO production without affecting the viability of RAW264.7. Pre-treatment of stimulated cells with columbin does not inhibit the translocation of NF-κB to the nucleus in LPS-stimulated cells. COX-1 and COX-2 inhibitory activities of columbin are 63.7±6.4% and 18.8±1.5% inhibition at 100μM, respectively. The interaction of columbin with Tyr385 and Arg120 signifies its higher activity in COX-2, as Tyr385 is reported to be involved in the abstraction of hydrogen from C-13 of arachidonate, and Arg120 is critical for high affinity arachidonate binding[1]. |
| In Vivo | Columbin inhibits oedema formation in mice paw. At doses of 300 mg/kg and 700 mg/kg, columbin inhibits inflammation from 0 to 5 h and the results are comparable to that of aspirin as a standard anti-inflammatory drug. The inhibitory effect of columbin on carrageenan induced paw oedema in mice may be due to the suppression of the release of mediators responsible for inflammation including prostaglandin[1]. Columbin is poorly bioavailable (2.8% p.o. and 14% i.p.) in rats, but its transport is rapid across the Caco-2 cell monolayers, suggesting that extensive first-pass metabolism in the liver is the likely reason for its poor bioavailability[2]. |
| Animal Admin | Rats: Male Wistar rats are treated as following: i.v. injection of columbin in EtOH and PEG-300 (1:1) is administrated through tail vein at dose of 20 mg/kg. Intraperitoneal (i.p.) injection of columbin in EtOH and PEG-300 (1:1) is administrated at dose of 20 mg/kg. An oral gavage of columbin suspended in oral suspension vehicle is given to rats at dose of 50 mg/kg. Blood samples (50-100 μL) are collected by snipping the tail into heparinized tubes at 0, 5, 15, 30, 45, 60, 120, 240, 360, 480 and 1440 min for i.v. administration, or at 0, 15, 30, 60,120, 180, 240, 360, 480, 1440 min for oral dosing or i.p. injection. The blood samples are stored at −20°C until analysis[2]. Mice: Male Balb/c mice (n=60) are randomly divided into six groups. Columbin is intra-peritoneally administered to mice at the dose of 30, 100, 300 and 700 mg/kg. Aspirin, an anti-inflammatory drug, is used as a positive control. To induce acute phase inflammation in paw, rats are injected subcutaneously into the right hind paw with a 1% solution of carrageenan dissolved in saline 30 min after vehicle or columbin treatment. The paw volumes are measured up to 5 h after the injection at intervals of 1 h. Paw volume is measured with a plethysmometer immediately prior to the injection of carrageenan and thereafter at an interval of 1 h for a period of 5 h[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 565.9±50.0 °C at 760 mmHg |
| Melting Point | 190-191ºC |
| Molecular Formula | C20H22O6 |
| Molecular Weight | 358.385 |
| Flash Point | 296.0±30.1 °C |
| Exact Mass | 358.141632 |
| PSA | 85.97000 |
| LogP | 0.58 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.593 |
| Storage condition | -20°C |
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
Columbin CAS#:546-97-4 |
| Literature: Yonemitsu, Michiko; Fukuda, Naomichi; Kimura, Takeatsu; Komori, Tetsuya Liebigs Annalen der Chemie, 1987 , p. 193 - 197 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
|
In vitro and in vivo anti-inflammatory activities of columbin through the inhibition of cycloxygenase-2 and nitric oxide but not the suppression of NF-κB translocation.
Eur. J. Pharmacol. 678(1-3) , 61-70, (2012) Columbin, a diterpenoid furanolactone, was isolated purely for the first time from the plant species Tinspora bakis. The anti-inflammatory effects of columbin were studied in vitro, in silico and in v... |
|
|
A bitter diterpenoid furanolactone columbin from Calumbae Radix inhibits azoxymethane-induced rat colon carcinogenesis.
Cancer Lett. 183(2) , 131-9, (2002) The modifying effect of dietary administration of a diterpenoid furanolactone columbin isolated from the crude drug Calumbae Radix (the root of Jateorhiza columba MIERS, Menispermacea) on azoxymethane... |
|
|
Inhibition of Naja nigricolis venom acidic phospholipase A2 catalysed hydrolysis of ghost red blood cells by columbin.
J. Enzyme Inhib. Med. Chem. 17(1) , 55-9, (2002) The inhibitory effects of a naturally occurring diterpenoid furanolactone, columbin, on partially purified acidic phospholipase A2 (PLA2) from Naja nigricolis was investigated. Columbin inhibited the ... |
| (1R,2S,3S,5S,8R,11R,12R)-5-(3-Furyl)-12-hydroxy-3,11-dimethyl-6,14-dioxatetracyclo[10.2.2.0.0]hexadec-15-ene-7,13-dione |
| 15,16-Epoxy-1b,4,12-trihydroxy-5,9-dimethyl-17,18-dinor-8bH,9bH,10a-labda-2,13(16),14-triene-19,20-dioic Acid 19,1:20,12-Dilactone |
| 1,4-Etheno-3H,7H-benzo[1,2-c:3,4-c']dipyran-3,7-dione, 9-(3-furanyl)-1,4,4a,5,6,6a,9,10,10a,10b-decahydro-4-hydroxy-4a,10a-dimethyl-, (1R,4R,4aR,6aR,9S,10aS,10bS)- |
| (2S,4aR,6aR,7R,10R,10aS,10bS)-2-(furan-3-yl)-7-hydroxy-6a,10b-dimethyl-1,2,4a,5,6,6a,7,10,10a,10b-decahydro-4H-10,7-(epoxymethano)benzo[f]isochromene-4,12-dione |
| Columbin |
![2-(furan-3-yl)-6a,10b-dimethyl-4,12-dioxo-1,4a,5,6,6a,10,10a,10b-octahydro-2h-10,7-(epoxymethano)benzo[f]isochromen-7(4h)-yl hexopyranoside structure](https://image.chemsrc.com/caspic/212/105597-94-2.png)
 CAS#:471-54-5
CAS#:471-54-5
