Violacein
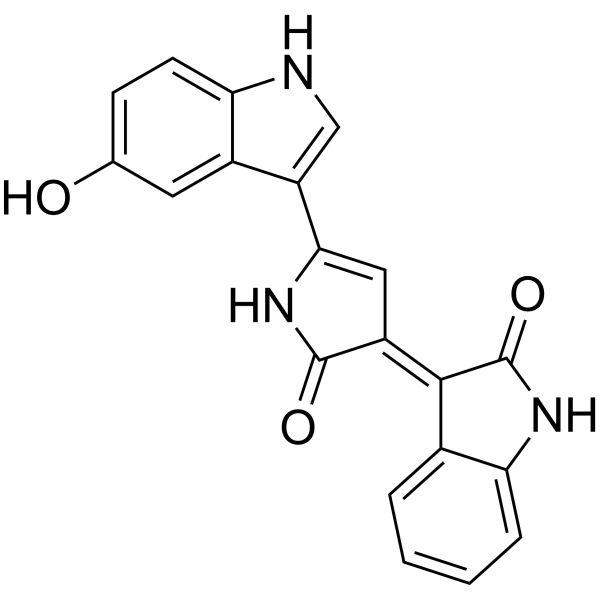
Violacein structure
|
Common Name | Violacein | ||
|---|---|---|---|---|
| CAS Number | 548-54-9 | Molecular Weight | 343.34 | |
| Density | 1.549g/cm3 | Boiling Point | 821.4ºC at 760mmHg | |
| Molecular Formula | C20H13N3O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 450.6ºC | |
Use of ViolaceinViolacein, a secondary metabolite produced by several microorganisms, possesses potent anticancer and low side effects. Violacein possesses antioxidant properties. Apoptosis inducer[1][2]. |
| Name | Violacein |
|---|---|
| Synonym | More Synonyms |
| Description | Violacein, a secondary metabolite produced by several microorganisms, possesses potent anticancer and low side effects. Violacein possesses antioxidant properties. Apoptosis inducer[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Microbial Metabolite |
| In Vitro | Violacein (0.25-3 µM; 24h; HCT116 and HT29 cells) possesses anticancer activity in both 2D and 3D cell models[1]. Violacein decreases RTKs expression and disturbs signaling pathways in CRC cells[1]. Cell Viability Assay[1]. Cell Line: HCT116 and HT29 (1.8 × 104 cells/well-100 µL) cells. Concentration: 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 µM. Incubation Time: 24h. Result: In the 2D culture model, the violacein treatment reduced the cell viability, since there was a decrease in formazan production in the HT29 and HCT116 cell lines. Moreover, HT29 was more sensitive to violacein, as evidenced by the IC50 value (0.6 μM) compared to HCT116 (1.2 μM). |
| References |
| Density | 1.549g/cm3 |
|---|---|
| Boiling Point | 821.4ºC at 760mmHg |
| Molecular Formula | C20H13N3O3 |
| Molecular Weight | 343.34 |
| Flash Point | 450.6ºC |
| Exact Mass | 343.09600 |
| PSA | 101.20000 |
| LogP | 3.11110 |
| Index of Refraction | 1.804 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The impact of plant volatiles on bacterial quorum sensing.
Lett. Appl. Microbiol. 60(1) , 8-19, (2015) Studies describing the use of essential oil constituents as antimicrobial agents have steadily increased; however, some phyto-constituents are often overlooked due to unfavourable minimum inhibitory c... |
|
|
Isolation of natural products by ion-exchange methods.
Methods Mol. Biol. 864 , 189-219, (2012) The primary goal of many natural products chemists is to extract, isolate, and characterize specific analytes from complex plant, animal, microbial, and food matrices. To achieve this goal, they rely ... |
|
|
Crystalline xylitol production by a novel yeast, Pichia caribbica (HQ222812), and its application for quorum sensing inhibition in gram-negative marker strain Chromobacterium violaceum CV026.
Appl. Biochem. Biotechnol. 169(6) , 1753-63, (2013) Xylitol, a sugar alcohol, is fast gaining ground over other artificial sugar substitutes owing to its advantageous properties. Xylitol is a safer alternative for diabetics because of insulin-independe... |
| (3Z)-3-[5-(5-hydroxy-1H-indol-3-yl)-2-oxo-1H-pyrrol-3-ylidene]-1H-indol-2-one |