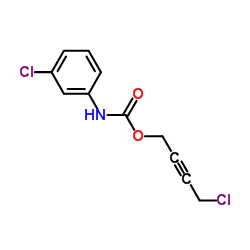
McN-A 343
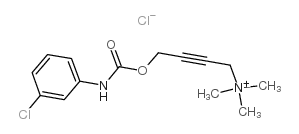
McN-A 343 structure
|
Common Name | McN-A 343 | ||
|---|---|---|---|---|
| CAS Number | 55-45-8 | Molecular Weight | 317.21100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C14H18Cl2N2O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of McN-A 343McN-A-343 is a selective M1 muscarinic agonist that stimulates muscarinic transmission in sympathetic ganglia. McN-A-343 reduces inflammation and oxidative stress in an experimental model of ulcerative colitis[1][2]. |
| Name | 4-[(3-chlorophenyl)carbamoyloxy]but-2-ynyl-trimethylazanium,chloride |
|---|---|
| Synonym | More Synonyms |
| Description | McN-A-343 is a selective M1 muscarinic agonist that stimulates muscarinic transmission in sympathetic ganglia. McN-A-343 reduces inflammation and oxidative stress in an experimental model of ulcerative colitis[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C14H18Cl2N2O2 |
|---|---|
| Molecular Weight | 317.21100 |
| Exact Mass | 316.07500 |
| PSA | 38.33000 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 24/25 |
| RIDADR | NONH for all modes of transport |
| RTECS | BR0318000 |
|
~% 
McN-A 343 CAS#:55-45-8 |
| Literature: Hopkins,T.R. et al. Journal of Organic Chemistry, 1962 , vol. 27, p. 659 - 662 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Central muscarinic cholinergic activation alters interaction between splenic dendritic cell and CD4+CD25- T cells in experimental colitis.
PLoS ONE 9(10) , e109272, (2014) The cholinergic anti-inflammatory pathway (CAP) is based on vagus nerve (VN) activity that regulates macrophage and dendritic cell responses in the spleen through alpha-7 nicotinic acetylcholine recep... |
|
|
Functional activation of G-proteins coupled with muscarinic acetylcholine receptors in rat brain membranes.
J. Pharmacol. Sci. 125(2) , 157-68, (2014) The functional activation of Gi/o proteins coupled to muscarinic acetylcholine receptors (mAChRs) was investigated with the conventional guanosine-5'-O-(3-[(35)S]thio) triphosphate ([(35)S]GTPγS) bind... |
|
|
Investigating the interaction of McN-A-343 with the M2 muscarinic receptor using its nitrogen mustard derivative.
Biochem. Pharmacol. 79(7) , 1025-35, (2010) We investigated whether the aziridinium ion formed from a nitrogen mustard derivative (4-[(2-bromoethyl)methyl-amino]-2-butynyl N-(3-chlorophenyl)carbamate; BR384) structurally related to McN-A-343 (4... |
| MCN A-343 chloride |
| McN-A-343 |
| A 343 |