Propentofylline
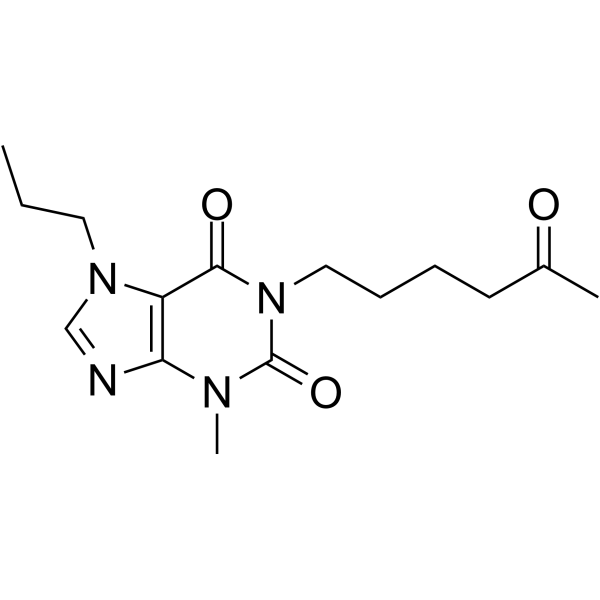
Propentofylline structure
|
Common Name | Propentofylline | ||
|---|---|---|---|---|
| CAS Number | 55242-55-2 | Molecular Weight | 306.360 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 541.4±56.0 °C at 760 mmHg | |
| Molecular Formula | C15H22N4O3 | Melting Point | 64-66ºC | |
| MSDS | Chinese USA | Flash Point | 281.2±31.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of PropentofyllinePropentofylline is a xanthine-derivative that inhibits adenosine uptake and blocks phosphodiesterase activity. Propentofylline has neuroprotective, antiproliferative, and anti-inflammatory effects that improve cognition and dementia severity in patients with Alzheimer's disease or vascular dementia. |
| Name | Propentofylline |
|---|---|
| Synonym | More Synonyms |
| Description | Propentofylline is a xanthine-derivative that inhibits adenosine uptake and blocks phosphodiesterase activity. Propentofylline has neuroprotective, antiproliferative, and anti-inflammatory effects that improve cognition and dementia severity in patients with Alzheimer's disease or vascular dementia. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 541.4±56.0 °C at 760 mmHg |
| Melting Point | 64-66ºC |
| Molecular Formula | C15H22N4O3 |
| Molecular Weight | 306.360 |
| Flash Point | 281.2±31.8 °C |
| Exact Mass | 306.169189 |
| PSA | 78.89000 |
| LogP | 1.38 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.602 |
| Storage condition | −20°C |
| Water Solubility | H2O: soluble12.4mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H332 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| RIDADR | NONH for all modes of transport |
| RTECS | UO8438700 |
| HS Code | 2933990090 |
|
~88% 
Propentofylline CAS#:55242-55-2 |
| Literature: Hoechst Aktiengesellschaft Patent: US4289776 A1, 1981 ; US 4289776 A |
|
~92% 
Propentofylline CAS#:55242-55-2 |
| Literature: Hoechst Aktiengesellschaft Patent: US5475002 A1, 1995 ; |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum.
Nat. Chem. Biol. 5 , 765-71, (2009) Studies of gene function and molecular mechanisms in Plasmodium falciparum are hampered by difficulties in characterizing and measuring phenotypic differences between individual parasites. We screened... |
|
|
Decreased extracellular adenosine levels lead to loss of hypoxia-induced neuroprotection after repeated episodes of exposure to hypoxia.
PLoS ONE 8(2) , e57065, (2013) Achieving a prolonged neuroprotective state following transient ischemic attacks (TIAs) is likely to effectively reduce the brain damage and neurological dysfunction associated with recurrent stroke. ... |
| 3-Methyl-1-(5-oxohexyl)-7-propyl-3,7-dihydro-1H-purine-2,6-dione |
| 1H-Purine-2,6-dione, 3,7-dihydro-3-methyl-1-(5-oxohexyl)-7-propyl- |
| Propentofylline |
| 3-methyl-1-(5-oxohexyl)-7-propylpurine-2,6-dione |
| Albert 285 |
| MFCD00133785 |
 CAS#:56395-62-1
CAS#:56395-62-1
