Lithium formate
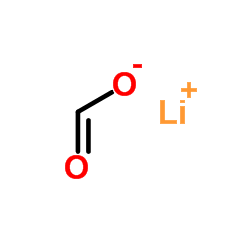
Lithium formate structure
|
Common Name | Lithium formate | ||
|---|---|---|---|---|
| CAS Number | 556-63-8 | Molecular Weight | 69.974 | |
| Density | 1.46g/cm3 | Boiling Point | 100.8ºC | |
| Molecular Formula | CH3LiO3 | Melting Point | 8.4ºC | |
| MSDS | N/A | Flash Point | 29.9ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | lithium formate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.46g/cm3 |
|---|---|
| Boiling Point | 100.8ºC |
| Melting Point | 8.4ºC |
| Molecular Formula | CH3LiO3 |
| Molecular Weight | 69.974 |
| Flash Point | 29.9ºC |
| Exact Mass | 70.024223 |
| PSA | 40.13000 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H319-H336 |
| Precautionary Statements | P280-P304 + P340 + P312-P305 + P351 + P338-P337 + P313 |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36/37/39 |
| RIDADR | UN1219 - class 3 - PG 2 - Isopropanol, solution |
|
~95% 
Lithium formate CAS#:556-63-8 |
| Literature: Jana, Anukul; Tavcar, Gasper; Roesky, Herbert W.; John, Michael Dalton Transactions, 2010 , vol. 39, # 40 p. 9487 - 9489 |
|
~% 
Lithium formate CAS#:556-63-8 |
| Literature: Spectrochimica Acta - Part A: Molecular and Biomolecular Spectroscopy, , vol. 66, # 3 p. 754 - 760 |
|
~% 
Lithium formate CAS#:556-63-8 |
| Literature: Angewandte Chemie - International Edition, , vol. 50, # 28 p. 6411 - 6414 |
|
~% 
Lithium formate CAS#:556-63-8 |
| Literature: Journal of the Indian Chemical Society, , vol. 67, # 1 p. 19 - 22 |
|
~% 
Lithium formate CAS#:556-63-8
Detail
|
| Literature: J. Gen. Chem. USSR (Engl. Transl.), , vol. 59, # 3.2 p. 687 - 691,607 - 610 |
|
~% 
Lithium formate CAS#:556-63-8
Detail
|
| Literature: J. Gen. Chem. USSR (Engl. Transl.), , vol. 59, # 3.2 p. 687 - 691,607 - 610 |
|
~% 
Lithium formate CAS#:556-63-8 |
| Literature: DE381957 ; Fortschr. Teerfarbenfabr. Verw. Industriezweige, vol. 14, p. 241 |
|
~% 
Lithium formate CAS#:556-63-8
Detail
|
| Literature: J. Gen. Chem. USSR (Engl. Transl.), , vol. 51, # 2 p. 299 - 302,239 - 242 |
|
~% 
Lithium formate CAS#:556-63-8
Detail
|
| Literature: J. Gen. Chem. USSR (Engl. Transl.), , vol. 51, # 2 p. 299 - 302,239 - 242 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
|
Bionanowhiskers from jute: preparation and characterization.
Carbohydr. Polym. 92(2) , 1116-23, (2013) Bionanowhiskers were extracted from jute by acid hydrolysis. At first cellulose microfibrils were formed by alkali treatment. Addition of an acid to the microfibrils triggered the formation of cellulo... |
|
|
Monoclonal antibodies selective for α-synuclein oligomers/protofibrils recognize brain pathology in Lewy body disorders and α-synuclein transgenic mice with the disease-causing A30P mutation.
J. Neurochem. 126(1) , 131-44, (2013) Inclusions of intraneuronal alpha-synuclein (α-synuclein) can be detected in brains of patients with Parkinson's disease and dementia with Lewy bodies. The aggregation of α-synuclein is a central feat... |
|
|
Density functional theory study on the adsorption and decomposition of the formic acid catalyzed by highly active mushroom-like Au@Pd@Pt tri-metallic nanoparticles.
Phys. Chem. Chem. Phys. 15(13) , 4625-33, (2013) Local structures and adsorption energies of a formic acid molecule and its decomposed intermediates (H, O, OH, CO, HCOO, and COOH) on highly electrocatalytically active mushroom-like Au-core@Pd-shell@... |
| Ameisensaeure,Lithiumformiat |
| formic acid,lithium formate |
| Formic acid, lithium salt, hydrate (1:1:1) |
| Lithiummethanoat |
| Lithiumformiatemonohydrate |
| Lithium formate hydrate (1:1:1) |
| lithium methanoate |
| Lithiumformiat |
| formic acid Li salt |
| Formic acid, lithium salt |
| Formic acid, lithium salt (1:1) |
| Formic acid lithium |
| Lithiumformiate-1-hydrate |
| Lithium formate |
| Einecs 209-133-0 |
| FORMIC ACID LITHIUM SALT |






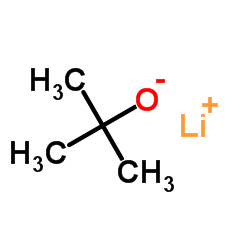
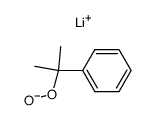
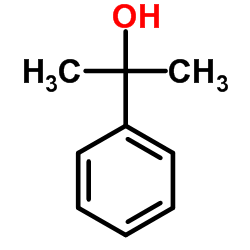
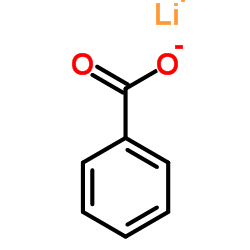
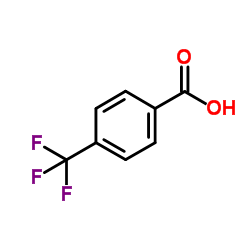 CAS#:455-24-3
CAS#:455-24-3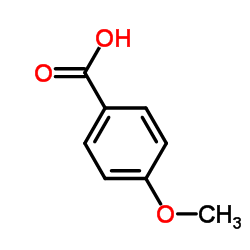 CAS#:100-09-4
CAS#:100-09-4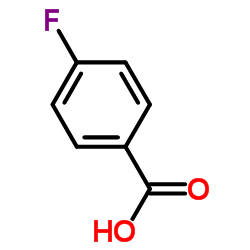 CAS#:456-22-4
CAS#:456-22-4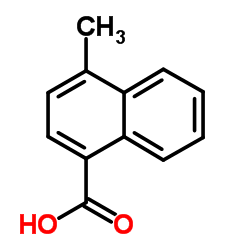 CAS#:4488-40-8
CAS#:4488-40-8 CAS#:445-29-4
CAS#:445-29-4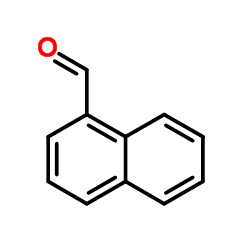 CAS#:66-77-3
CAS#:66-77-3 CAS#:92-92-2
CAS#:92-92-2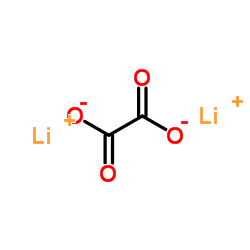 CAS#:553-91-3
CAS#:553-91-3 CAS#:67-56-1
CAS#:67-56-1