CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
SP1880000
-
CHEMICAL NAME :
-
Phenothiazine, 10-(2-(1-methyl-2-piperidyl)ethyl)-2-(methylsulfinyl) -
-
CAS REGISTRY NUMBER :
-
5588-33-0
-
LAST UPDATED :
-
199512
-
DATA ITEMS CITED :
-
10
-
MOLECULAR FORMULA :
-
C21-H26-N2-O-S2
-
MOLECULAR WEIGHT :
-
386.61
-
WISWESSER LINE NOTATION :
-
T C666 BN ISJ ESO&1 B2- BT6NTJ A
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
86 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - arrhythmias (including changes in conduction)
-
REFERENCE :
-
JCPYDR Journal of Clinical Pyschopharmacology. (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21202) V.1- 1981- Volume(issue)/page/year: 2,222,1982
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
42 mg/kg
-
TOXIC EFFECTS :
-
Vascular - regional or general arteriolar or venous dilation Lungs, Thorax, or Respiration - chronic pulmonary edema Blood - hemorrhage
-
REFERENCE :
-
ARGPAQ Archives of General Psychiatry. (AMA, 535 N. Dearborn St., Chicago,IL 60610) V.3- 1960- Volume(issue)/page/year: 34,955,1977
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
644 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 19,363,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
509 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 19,363,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
560 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
27ZQAG "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972 Volume(issue)/page/year: -,29,1972
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
26 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
27ZQAG "Psychotropic Drugs and Related Compounds," 2nd ed., Usdin, E., and D.H. Efron, Washington, DC, 1972 Volume(issue)/page/year: -,29,1972
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
800 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 19,363,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
405 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 19,363,1971 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
60480 mg/kg/72W-I
-
TOXIC EFFECTS :
-
Brain and Coverings - recordings from specific areas of CNS Skin and Appendages - dermatitis, other (after systemic exposure) Related to Chronic Data - death
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 19,363,1971 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X4738 No. of Facilities: 7 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 103 (estimated) No. of Female Employees: 52 (estimated)
|
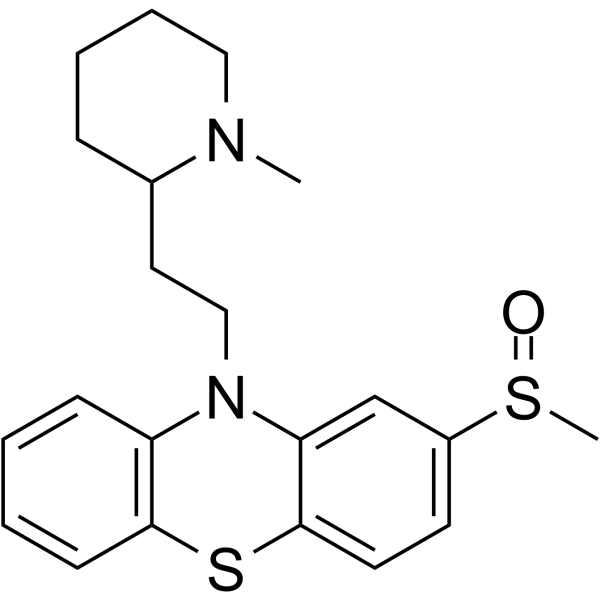

 CAS#:14759-06-9
CAS#:14759-06-9