Cefuroxime sodium
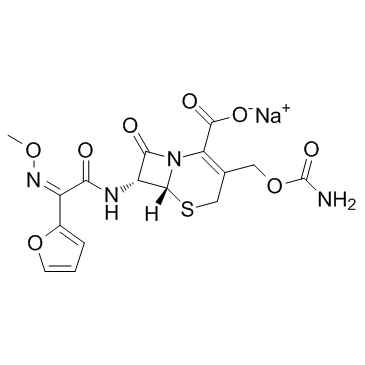
Cefuroxime sodium structure
|
Common Name | Cefuroxime sodium | ||
|---|---|---|---|---|
| CAS Number | 56238-63-2 | Molecular Weight | 446.367 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H15N4NaO8S | Melting Point | 240-245°C(dec) | |
| MSDS | USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Cefuroxime sodiumCefuroxime sodium is an enteral or oral second-generation cephalosporin antibiotic. |
| Name | Cefuroxime Sodium Salt |
|---|---|
| Synonym | More Synonyms |
| Description | Cefuroxime sodium is an enteral or oral second-generation cephalosporin antibiotic. |
|---|---|
| Related Catalog |
| Melting Point | 240-245°C(dec) |
|---|---|
| Molecular Formula | C16H15N4NaO8S |
| Molecular Weight | 446.367 |
| Exact Mass | 446.050842 |
| PSA | 201.89000 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317 |
| Precautionary Statements | P280 |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R42/43 |
| Safety Phrases | S22-S36/37-S45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | XI0330000 |
| HS Code | 2941905990 |
| HS Code | 2941905990 |
|---|
|
Pharmacokinetics of cefuroxime in porcine cortical and cancellous bone determined by microdialysis.
Antimicrob. Agents Chemother. 58(6) , 3200-5, (2014) Traditionally, the pharmacokinetics of antimicrobials in bone have been investigated using bone biopsy specimens, but this approach suffers from considerable methodological limitations. Consequently, ... |
|
|
18-Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography scan for monitoring the therapeutic response in experimental Staphylococcus aureus foreign-body osteomyelitis.
J. Orthop. Surg. Res. 10 , 132, (2015) 18-Fluoro-2-deoxy-D-glucose positron emission tomography combined with computed tomography ((18)F-FDG PET/CT) scan is useful for diagnosis of osteoarticular infections. Whether (18)F-FDG PET/CT scanni... |
|
|
Gut-liver axis improves with meloxicam treatment after cirrhotic liver resection.
World J. Gastroenterol. 20(40) , 14841-54, (2014) To investigate the effect of meloxicam on the gut-liver axis after cirrhotic liver resection.Forty-four male Wistar rats were assigned to three groups: (1) control group (CG); (2) bile duct ligation w... |
| MFCD00079036 |
| EINECS 260-073-1 |
| Biociclin |
| Cefuroxime sodium |
| 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(aminocarbonyl)oxy]methyl]-7-[[(2Z)-2-(2-furanyl)-2-(methoxyimino)-1-oxoethyl]amino]-8-oxo-, sodium salt, (6R,7R)- (1:1) |
| Cefuroxime Sodium Salt |
| Zinacef |
| sodium (6R,7R)-3-[(carbamoyloxy)methyl]-7-{[(2Z)-2-(furan-2-yl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| Curoxim |
| Sodium (6R,7R)-3-[(carbamoyloxy)methyl]-7-{[(2Z)-2-(2-furyl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate |
| SODIUM CEFUROXIME |
| Cefuroxime (sodium) |

