Bromonitromethane
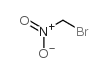
Bromonitromethane structure
|
Common Name | Bromonitromethane | ||
|---|---|---|---|---|
| CAS Number | 563-70-2 | Molecular Weight | 139.93600 | |
| Density | 2.07 | Boiling Point | 146-148 °C (750 mmHg) | |
| Molecular Formula | CH2BrNO2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 4°C | |
| Symbol |


GHS03, GHS07 |
Signal Word | Danger | |
Use of BromonitromethaneBromonitromethane is a halonitromethane that can be used in production of various protected α-bromo nitroalkane donors for use in Umpolung Amide Synthesis (UmAS)[1][2]. |
| Name | Bromonitromethane |
|---|---|
| Synonym | More Synonyms |
| Description | Bromonitromethane is a halonitromethane that can be used in production of various protected α-bromo nitroalkane donors for use in Umpolung Amide Synthesis (UmAS)[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 2.07 |
|---|---|
| Boiling Point | 146-148 °C (750 mmHg) |
| Molecular Formula | CH2BrNO2 |
| Molecular Weight | 139.93600 |
| Flash Point | 4°C |
| Exact Mass | 138.92700 |
| PSA | 45.82000 |
| LogP | 1.13870 |
| Index of Refraction | 1.485-1.495 |
| Symbol |


GHS03, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H272-H315-H319-H335 |
| Precautionary Statements | P220-P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | O: Oxidizing agent;Xn: Harmful; |
| Risk Phrases | R8;R36/37/38 |
| Safety Phrases | S36/37/39-S26-S17-S36 |
| RIDADR | 3098 |
| RTECS | PA5360000 |
| Packaging Group | III |
| Hazard Class | 5.1 |
| HS Code | 2904909090 |
| HS Code | 2904909090 |
|---|---|
| Summary | HS:2904909090 sulphonated, nitrated or nitrosated derivatives of hydrocarbons, whether or not halogenated VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Silyl imine electrophiles in enantioselective catalysis: a Rosetta Stone for peptide homologation, enabling diverse N-protected aryl glycines from aldehydes in three steps.
Org. Lett. , (2014) We report that N-(trimethylsilyl)imines serve in the Bis(AMidine)-catalyzed addition of bromonitromethane with a high degree of enantioselection. This allows for the production of a range of protected... |
|
|
Stereoselective synthesis of highly functionalized nitrocyclopropanes through the organocatalyic Michael-addition-initiated cyclization of bromonitromethane and β,γ-unsaturated α-ketoesters.
Chem. Asian J. 8(11) , 2859-63, (2013) A highly diastereo- and enantioselective cyclopropanation of β,γ-unsaturated α-ketoesters with bromonitromethane has been successfully developed through a domino Michael-addition/intramolecular-alkyla... |
|
|
Synthesis of enantiopure 2-C-glycosyl-3-nitrochromenes.
J. Org. Chem. 78(24) , 12831-6, (2013) A novel methodology has been developed to obtain enantiopure 2-C-glycosyl-3-nitrochromenes. First, (Z)-1-bromo-1-nitroalkenes were prepared from the corresponding sugar aldehydes through a sodium iodi... |
| MFCD00007401 |
| EINECS 209-258-0 |
| bromo(nitro)methane |

