Doxycycline
Modify Date: 2024-01-02 08:06:04

Doxycycline structure
|
Common Name | Doxycycline | ||
|---|---|---|---|---|
| CAS Number | 564-25-0 | Molecular Weight | 444.435 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 685.2±55.0 °C at 760 mmHg | |
| Molecular Formula | C22H24N2O8 | Melting Point | 206-209ºC | |
| MSDS | N/A | Flash Point | 368.2±31.5 °C | |
Use of DoxycyclineDoxycycline, an antibiotic, is an orally active and broad-spectrum metalloproteinase (MMP) inhibitor[1]. |
| Name | doxycycline |
|---|---|
| Synonym | More Synonyms |
| Description | Doxycycline, an antibiotic, is an orally active and broad-spectrum metalloproteinase (MMP) inhibitor[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Doxycycline affects growth of glioma cells only under high concentrations[2]. Doxycycline decreases MT-CO1 protein content with concentrations of 1 µg/mL and higher in SVG cells[2]. Cell Proliferation Assay[2] Cell Line: LNT-229, G55, and U343 glioma cells Concentration: 0.01, 0.1, 1, or 10 µg/mL Incubation Time: 4 days Result: Affected growth of glioma cells only under high concentrations. Western Blot Analysis[2] Cell Line: SVG cells Concentration: 0.01, 0.1, 1, or 10 µg/mL Incubation Time: 24 hours Result: Decreaseed MT-CO1 protein content with concentrations of 1 µg/mL and higher. |
| In Vivo | Doxycycline (oral; 200 or 800 mg/kg/day; for 3 months) reduces active MMP-9 in untreated HT mice in a dose-dependent manner[1]. Animal Model: 6-month-old female Heterozygous Col3a1-deficient (HT) mice[1] Dosage: 200 or 800 mg/kg Administration: Oral; per day; for 3 months Result: Reduced active MMP-9 in a dose-dependent manner. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 685.2±55.0 °C at 760 mmHg |
| Melting Point | 206-209ºC |
| Molecular Formula | C22H24N2O8 |
| Molecular Weight | 444.435 |
| Flash Point | 368.2±31.5 °C |
| Exact Mass | 444.153259 |
| PSA | 181.62000 |
| LogP | 1.36 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.737 |
| Storage condition | 2-8C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| HS Code | 3004909090 |
|---|
|
~98% 
Doxycycline CAS#:564-25-0 |
| Literature: Tetrahedron Letters, , vol. 34, # 9 p. 1471 - 1474 |
|
~% 
Doxycycline CAS#:564-25-0 |
| Literature: CrystEngComm, , vol. 14, # 7 p. 2532 - 2540 |
| HS Code | 3004909090 |
|---|
| Monodox |
| azudoxat |
| Doxycen |
| MFCD02682958 |
| gs-3065 |
| (2Z,4S,4aR,5S,5aR,6R,12aS)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5a,6,12a-tetrahydrotetracene-1,3,12(2H,4H,5H)-trione |
| (2Z,4S,4aR,5S,5aR,6R,12aS)-2-[Amino(hydroxy)methylene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5a,6,12a-tetrahydro-1,3,12(2H,4H,5H)-tetracenetrione |
| 1,3,12(2H,4H,5H)-Naphthacenetrione, 2-(aminohydroxymethylene)-4-(dimethylamino)-4a,5a,6,12a-tetrahydro-5,10,11,12a-tetrahydroxy-6-methyl-, (2Z,4S,4aR,5S,5aR,6R,12aS)- |
| Ronaxan |
| doxocycline |
| doryx |
| Unidox |
| (2Z,4S,4aR,5S,5aR,6R,12aS)-2-[Amino(hydroxy)methylene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5a,6,12a-tetrahydrotetracene-1,3,12(2H,4H,5H)-trione |
| doxitard |
| Doxycycline |
| spanor |
| Doxinyl |
| EINECS 209-271-1 |
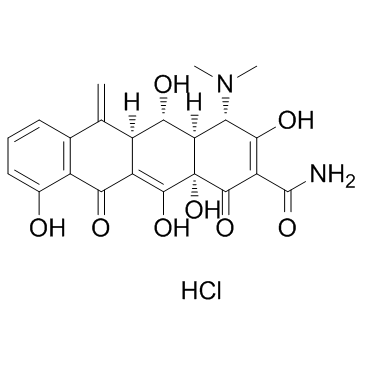
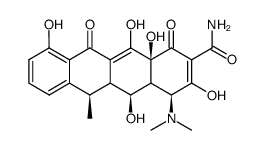
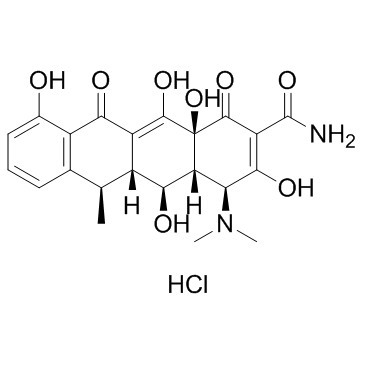
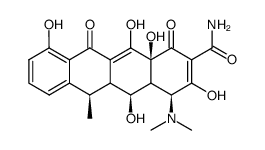 CAS#:6543-77-7
CAS#:6543-77-7
