Rhamnocitrin
Modify Date: 2024-01-07 13:50:39
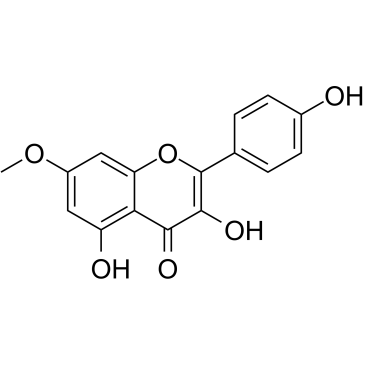
Rhamnocitrin structure
|
Common Name | Rhamnocitrin | ||
|---|---|---|---|---|
| CAS Number | 569-92-6 | Molecular Weight | 300.263 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 571.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H12O6 | Melting Point | 225-227ºC | |
| MSDS | N/A | Flash Point | 218.4±23.6 °C | |
Use of RhamnocitrinRhamnocitrin is a flavonoid isolated from astragalus complanatus R. Br. (Sha-yuan-zi)[1]. Rhamnocitrin is a scavenger of DPPH with an IC50 of 28.38 mM. Rhamnocitrin has anti-oxidant, anti-inflammatory and an-tiatherosclerosis activity[2]. |
| Name | rhamnocitrin |
|---|---|
| Synonym | More Synonyms |
| Description | Rhamnocitrin is a flavonoid isolated from astragalus complanatus R. Br. (Sha-yuan-zi)[1]. Rhamnocitrin is a scavenger of DPPH with an IC50 of 28.38 mM. Rhamnocitrin has anti-oxidant, anti-inflammatory and an-tiatherosclerosis activity[2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 571.9±50.0 °C at 760 mmHg |
| Melting Point | 225-227ºC |
| Molecular Formula | C16H12O6 |
| Molecular Weight | 300.263 |
| Flash Point | 218.4±23.6 °C |
| Exact Mass | 300.063385 |
| PSA | 100.13000 |
| LogP | 2.56 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.710 |
| Hazard Codes | Xi |
|---|
| 3,5,4'-trihydroxy-7-methoxyflavone |
| Rhamnocitrin |
| 3,4',5-Trihydroxy-7-methoxyflavone |
| 3,5-Dihydroxy-2-(4-hydroxyphenyl)-7-methoxy-4H-chromen-4-one |
| 5,4'-dihydroxy-7-methoxyflavonol |
| kaempferol 7-O-methyl ether |
| 4H-1-Benzopyran-4-one, 3,5-dihydroxy-2-(4-hydroxyphenyl)-7-methoxy- |
| 7-Methylkaempferol |
| 3,5-dihydroxy-2-(4-hydroxyphenyl)-7-methoxychromen-4-one |
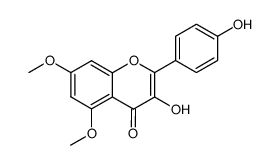 CAS#:77316-40-6
CAS#:77316-40-6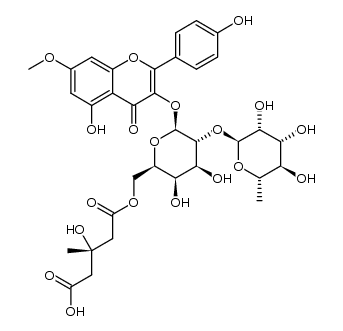 CAS#:1391144-80-1
CAS#:1391144-80-1 CAS#:1391144-89-0
CAS#:1391144-89-0![5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one Structure](https://image.chemsrc.com/caspic/457/57525-01-6.png) CAS#:57525-01-6
CAS#:57525-01-6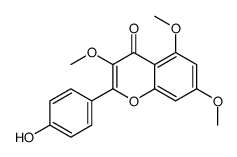 CAS#:59259-80-2
CAS#:59259-80-2 CAS#:520-18-3
CAS#:520-18-3