Ethynyl estradiol
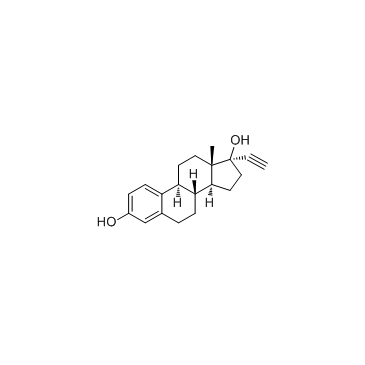
Ethynyl estradiol structure
|
Common Name | Ethynyl estradiol | ||
|---|---|---|---|---|
| CAS Number | 57-63-6 | Molecular Weight | 296.403 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 457.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C20H24O2 | Melting Point | 182-183 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 211.2±23.3 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of Ethynyl estradiolEthynyl estradiol is an orally bio-active estrogen used in almost all modern formulations of combined oral contraceptive pills.Target: Estrogen ReceptorEthinyl estradiol (EE), also sometimes written as ethinylestradiol, ethynyl estradiol, or ethinyl estradiol, is a derivative of 17β-estradiol (E2), the major endogenous estrogen in humans. EE is an orally bioactive estrogen used in many formulations of combined oral contraceptive pills. It is one of the most commonly used medications for this purpose. Transdermal ethinyl estradiol carries a greater risk of clot formation and venous thromboembolism than 17 beta estradiol, which some have theorized to be related to different amounts of hepatic metabolism after absorption. The same contraindications and precautions apply for EE as with other estrogen medications.Estinyl was a preparation of EE alone that was used for the management of menopausal symptoms and female hypogonadism. EE is released into the environment as a xenoestrogen from the urine and feces of people who take it as a medication. The major concern with unopposed estrogen is of endometrial cancer. As such, the medication is generally prescribed with progesterone in the setting of birth control. The first orally active semisynthetic steroidal estrogen, EE (17α-ethynylestradiol), the 17α-ethynyl analog of E2, was synthesized in 1938 by Hans Herloff Inhoffen and Walter Hohlweg at Schering AG in Berlin. |
| Name | 17α-ethynylestradiol |
|---|---|
| Synonym | More Synonyms |
| Description | Ethynyl estradiol is an orally bio-active estrogen used in almost all modern formulations of combined oral contraceptive pills.Target: Estrogen ReceptorEthinyl estradiol (EE), also sometimes written as ethinylestradiol, ethynyl estradiol, or ethinyl estradiol, is a derivative of 17β-estradiol (E2), the major endogenous estrogen in humans. EE is an orally bioactive estrogen used in many formulations of combined oral contraceptive pills. It is one of the most commonly used medications for this purpose. Transdermal ethinyl estradiol carries a greater risk of clot formation and venous thromboembolism than 17 beta estradiol, which some have theorized to be related to different amounts of hepatic metabolism after absorption. The same contraindications and precautions apply for EE as with other estrogen medications.Estinyl was a preparation of EE alone that was used for the management of menopausal symptoms and female hypogonadism. EE is released into the environment as a xenoestrogen from the urine and feces of people who take it as a medication. The major concern with unopposed estrogen is of endometrial cancer. As such, the medication is generally prescribed with progesterone in the setting of birth control. The first orally active semisynthetic steroidal estrogen, EE (17α-ethynylestradiol), the 17α-ethynyl analog of E2, was synthesized in 1938 by Hans Herloff Inhoffen and Walter Hohlweg at Schering AG in Berlin. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
[1]. Djerassi C. Chemical birth of the pill. 1992. Am J Obstet Gynecol. 2006 Jan;194(1):290-8. |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 457.2±45.0 °C at 760 mmHg |
| Melting Point | 182-183 °C(lit.) |
| Molecular Formula | C20H24O2 |
| Molecular Weight | 296.403 |
| Flash Point | 211.2±23.3 °C |
| Exact Mass | 296.177643 |
| PSA | 40.46000 |
| LogP | 4.52 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.624 |
| Storage condition | -20°C Freezer |
| Water Solubility | ethanol: 50 mg/mL, clear, slightly yellow |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H350 |
| Precautionary Statements | P201-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R45;R22 |
| Safety Phrases | S53-S36/37/39-S45 |
| RIDADR | 2811.0 |
| WGK Germany | 3 |
| RTECS | RC8925000 |
| HS Code | 2937290090 |
|
~78% 
Ethynyl estradiol CAS#:57-63-6 |
| Literature: Rao, Pemmaraju N.; Cessac, James W.; Kim, Hyun K. Steroids, 1994 , vol. 59, # 11 p. 621 - 627 |
|
~% 
Ethynyl estradiol CAS#:57-63-6 |
| Literature: US2265976 , ; DE681869 , ; DRP/DRBP Org.Chem. |
|
~% 
Ethynyl estradiol CAS#:57-63-6 |
| Literature: Pharmazie, , vol. 39, # 1 p. 27 - 29 |
|
~% 
Ethynyl estradiol CAS#:57-63-6 |
| Literature: FR809819 , ; |
|
~% 
Ethynyl estradiol CAS#:57-63-6 |
| Literature: Chemische Berichte, , vol. 77/79, p. 121,125 |
|
~% 
Ethynyl estradiol CAS#:57-63-6 |
| Literature: Chemische Berichte, , vol. 77/79, p. 121,125 |
|
~% 
Ethynyl estradiol CAS#:57-63-6
Detail
|
| Literature: Steroids, , vol. 36, # 3 p. 255 - 282 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2937290090 |
|---|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
|
|
The Japanese toxicogenomics project: application of toxicogenomics.
Mol. Nutr. Food. Res. 54 , 218-27, (2010) Biotechnology advances have provided novel methods for the risk assessment of chemicals. The application of microarray technologies to toxicology, known as toxicogenomics, is becoming an accepted appr... |
| Estinyl |
| Prosexol |
| diprol |
| Gynolett |
| eed |
| LYNORAL |
| Estigyn |
| Dufaston |
| Estra-1,3,5(10)-triene-3,17β-diol, 17α-ethynyl- |
| Kolpolyn |
| MFCD00003690 |
| Estra-1,3,5(10)-triene-3,17-diol, 17-ethynyl-, (17β)- |
| Anovlar |
| Ertonyl |
| Ethynyl estradiol |
| Estoral |
| Estra-1,3,5[10]-triene-3,17β-diol, 17-ethynyl- |
| Esteed |
| Ethinyl estradiol |
| (17α)-19-Norpregna-1(10),2,4-trien-20-yne-3,17-diol |
| (8R,9S,13S,14S,17R)-17-Ethinyl-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-3,17-diol |
| 17α-ethynylestradiol |
| Progylut |
| Ethynylestradiol |
| Ethinyloestradiol |
| (8R,9S,13S,14S,17R)-17-éthynyl-13-méthyl-7,8,9,11,12,13,14,15,16,17-décahydro-6H-cyclopenta[a]phénanthrène-3,17-diol |
| (17α)-19-Norpregna-1,3,5(10)-trien-20-yne-3,17-diol |
| Etinilestradiol |
| 17alpha-ethynylestradiol |
| (17b)-17-ethynylestra-1(10),2,4-triene-3,17-diol |
| 17-Ethynyloestra-1,3,5(10)-triene-3,17β-diol |
| Estra-1,3,5(10)-triene-3,17β-diol, 17-ethynyl- |
| EINECS 200-342-2 |
| Ylestrol |
| Progynon M |
| estra-1(10),2,4-triene-3,17-diol, 17-ethynyl-, (17b)- |
| Ethinyl estradiol (USP) |
| Etivex |
| 17a-Ethynyl-1,3,5(10)-estratriene-3,17b-diol |
| Ethinyl Estradial |
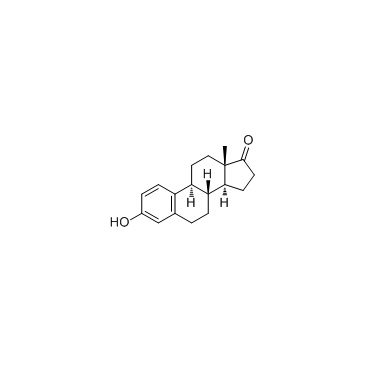
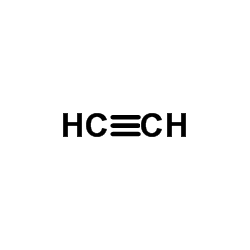
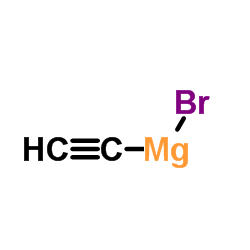
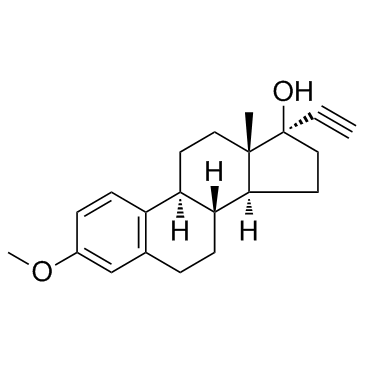
![(8S,9S,13S,14S)-17-ethynyl-3-methoxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthrene-2,17-diol structure](https://image.chemsrc.com/caspic/269/26011-40-5.png)
 CAS#:60134-76-1
CAS#:60134-76-1 CAS#:75803-36-0
CAS#:75803-36-0 CAS#:24560-70-1
CAS#:24560-70-1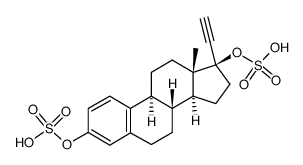 CAS#:75803-37-1
CAS#:75803-37-1 CAS#:75803-39-3
CAS#:75803-39-3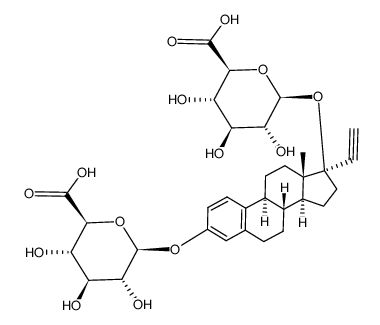 CAS#:75812-41-8
CAS#:75812-41-8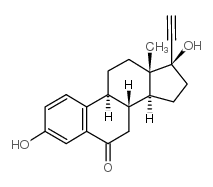 CAS#:38002-18-5
CAS#:38002-18-5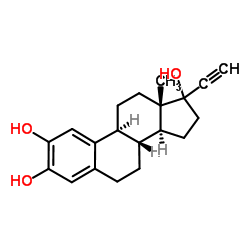 CAS#:50394-89-3
CAS#:50394-89-3 CAS#:50394-90-6
CAS#:50394-90-6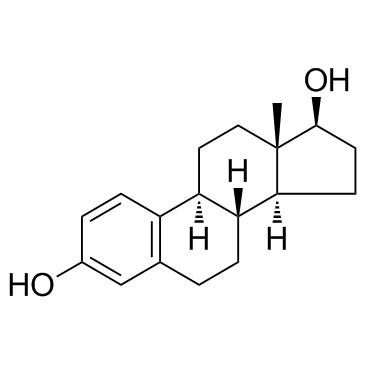 CAS#:50-28-2
CAS#:50-28-2
