N-Boc-piperazine
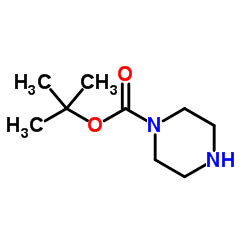
N-Boc-piperazine structure
|
Common Name | N-Boc-piperazine | ||
|---|---|---|---|---|
| CAS Number | 57260-71-6 | Molecular Weight | 186.251 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 258.0±15.0 °C at 760 mmHg | |
| Molecular Formula | C9H18N2O2 | Melting Point | 47-49ºC | |
| MSDS | Chinese USA | Flash Point | 109.8±20.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of N-Boc-piperazineN-Boc-piperazine is a Alkyl/ether-based PROTAC linker that can be used in the synthesis of PROTAC PD-1/PD-L1 degrader-1 (HY-131183)[1]. |
| Name | 1-Boc-piperazine |
|---|---|
| Synonym | More Synonyms |
| Description | N-Boc-piperazine is a Alkyl/ether-based PROTAC linker that can be used in the synthesis of PROTAC PD-1/PD-L1 degrader-1 (HY-131183)[1]. |
|---|---|
| Related Catalog | |
| Target |
Alkyl/ether |
| In Vitro | PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[1]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 258.0±15.0 °C at 760 mmHg |
| Melting Point | 47-49ºC |
| Molecular Formula | C9H18N2O2 |
| Molecular Weight | 186.251 |
| Flash Point | 109.8±20.4 °C |
| Exact Mass | 186.136826 |
| PSA | 41.57000 |
| LogP | 0.55 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.467 |
| Storage condition | Refrigerator |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis and dual D2 and 5-HT1A receptor binding affinities of 5-piperidinyl and 5-piperazinyl-1H-benzo[d]imidazol-2(3H)-ones.
J. Enzyme Inhib. Med. Chem. , (2013) A series of new 5-piperidinyl and 5-piperazinyl-1H-benzo[d]imidazol-2(3H)-ones have been synthesized and evaluated for dual D2 and 5-HT1A receptor binding affinities. The synthesized ligands are struc... |
|
|
Design, synthesis and structure-activity relationship studies of novel indazole analogues as DNA gyrase inhibitors with Gram-positive antibacterial activity.
Bioorg. Med. Chem. Lett. 14 , 2857-2862, (2004) In this study, we report the design, synthesis and structure-activity relationships of novel indazole derivatives as DNA gyrase inhibitors with Gram-positive antibacterial activity. Our results show t... |
|
|
Molecular iodine-catalyzed facile procedure for N-Boc protection of amines.
J. Org. Chem. 71 , 8283, (2006) An efficient and practical protocol for the protection of various structurally and electronically divergent aryl and aliphatic amines using (Boc)2O in the presence of a catalytic amount of molecular i... |
| piperazine-1-carboxylic acid tert-butyl ester |
| 1-Piperazinecarboxylic acid, 1,1-dimethylethyl ester |
| BOC-PIPERAZINE |
| 2-Methyl-2-propanyl 1-piperazinecarboxylate |
| 1-Boc piperzine |
| t-Butyl 1-piperazincarboxylate |
| tert-butyl piperazine-1-carboxylate |
| 1,1-Dimethylethyl 1-piperazinecarboxylate |
| 1-BOC-Piperazine |
| PIBOC |
| MFCD00075265 |
| tert-butyl tetrahydropyrazine-1(2H)-carboxylate |
| N-BOC-PIPERAZINE |
| tert-butyl l-piperazinecarboxylate |
| 1-N-BOC-PIPERAZINE |
| RARECHEM AR PA 0026 |
| BOC-PAZ |
| N-Boc-piperazine; tert-butyl piperazine-1-carboxylate |
| tert-Butyl 1-piperazinecarboxylate |
| N-Boc-piperazine |
| N Boc-Piperazine |
 CAS#:110-85-0
CAS#:110-85-0 CAS#:24424-99-5
CAS#:24424-99-5 CAS#:164331-38-8
CAS#:164331-38-8 CAS#:219509-79-2
CAS#:219509-79-2 CAS#:57260-70-5
CAS#:57260-70-5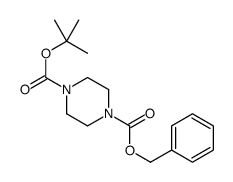 CAS#:121370-60-3
CAS#:121370-60-3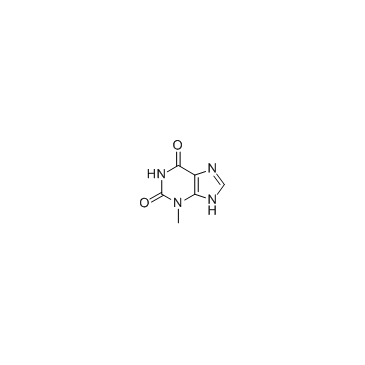 CAS#:1076-22-8
CAS#:1076-22-8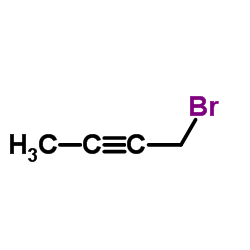 CAS#:3355-28-0
CAS#:3355-28-0 CAS#:85-18-7
CAS#:85-18-7![4-(4-Boc-1-piperazinyl)-5-bromo-7H-pyrrolo[2,3-d]pyrimidine structure](https://image.chemsrc.com/caspic/185/1072027-36-1.png) CAS#:1072027-36-1
CAS#:1072027-36-1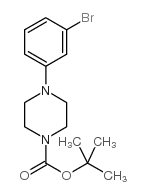 CAS#:327030-39-7
CAS#:327030-39-7 CAS#:38212-33-8
CAS#:38212-33-8 CAS#:497915-42-1
CAS#:497915-42-1 CAS#:40172-95-0
CAS#:40172-95-0 CAS#:374930-88-8
CAS#:374930-88-8 CAS#:39512-50-0
CAS#:39512-50-0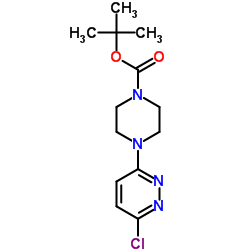 CAS#:492431-11-5
CAS#:492431-11-5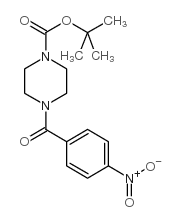 CAS#:509073-62-5
CAS#:509073-62-5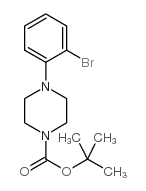 CAS#:494773-35-2
CAS#:494773-35-2
