Equisetin
Modify Date: 2024-01-03 15:04:33
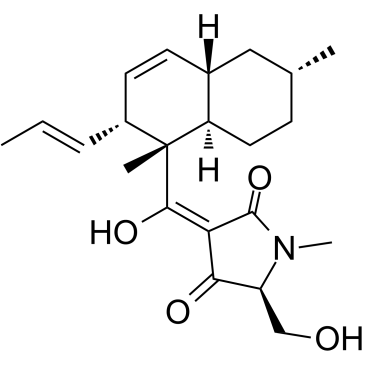
Equisetin structure
|
Common Name | Equisetin | ||
|---|---|---|---|---|
| CAS Number | 57749-43-6 | Molecular Weight | 373.48600 | |
| Density | 1.187 g/cm3 | Boiling Point | 513.6ºC at 760 mmHg | |
| Molecular Formula | C22H31NO4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 264.4ºC | |
Use of EquisetinEquisetin is an N-methylserine-derived acyl tetramic acid isolated from a terrestrial fungus Fusarium equiseti NRRL 5537[1]. Equisetin is a tetramate-containing natural product with antibiotic and cytotoxic activity[2]. Equisetin inhibits the growth of Gram-positive bacteria and HIV-1 integrase activity but shows no activity against Gram-negative bacteria[3]. Equisetin is a Quorum-sensing inhibitor (QSI) that attenuates QS-regulated virulence phenotypes in P. aeruginosa without affecting the growth of bacterias, serves as a leading compound for the treatment of P. aeruginosa infections[4]. |
| Name | (3E,5S)-3-[[(1S,2R,4aS,6R,8aR)-1,6-dimethyl-2-[(E)-prop-1-enyl]-4a,5,6,7,8,8a-hexahydro-2H-naphthalen-1-yl]-hydroxymethylidene]-5-(hydroxymethyl)-1-methylpyrrolidine-2,4-dione |
|---|---|
| Synonym | More Synonyms |
| Description | Equisetin is an N-methylserine-derived acyl tetramic acid isolated from a terrestrial fungus Fusarium equiseti NRRL 5537[1]. Equisetin is a tetramate-containing natural product with antibiotic and cytotoxic activity[2]. Equisetin inhibits the growth of Gram-positive bacteria and HIV-1 integrase activity but shows no activity against Gram-negative bacteria[3]. Equisetin is a Quorum-sensing inhibitor (QSI) that attenuates QS-regulated virulence phenotypes in P. aeruginosa without affecting the growth of bacterias, serves as a leading compound for the treatment of P. aeruginosa infections[4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.187 g/cm3 |
|---|---|
| Boiling Point | 513.6ºC at 760 mmHg |
| Molecular Formula | C22H31NO4 |
| Molecular Weight | 373.48600 |
| Flash Point | 264.4ºC |
| Exact Mass | 373.22500 |
| PSA | 77.84000 |
| LogP | 2.95920 |
| Index of Refraction | 1.587 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| Equisetin |
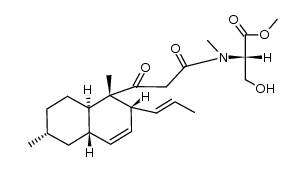 CAS#:122902-79-8
CAS#:122902-79-8 CAS#:122902-71-0
CAS#:122902-71-0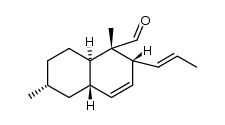 CAS#:122902-74-3
CAS#:122902-74-3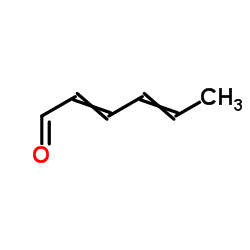 CAS#:142-83-6
CAS#:142-83-6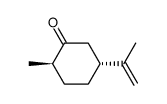 CAS#:5524-05-0
CAS#:5524-05-0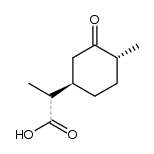 CAS#:38339-60-5
CAS#:38339-60-5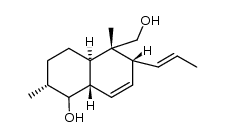 CAS#:122902-65-2
CAS#:122902-65-2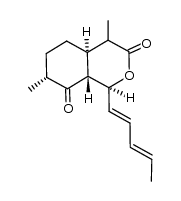 CAS#:122902-57-2
CAS#:122902-57-2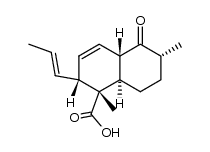 CAS#:122902-61-8
CAS#:122902-61-8