Hydrochlorothiazide
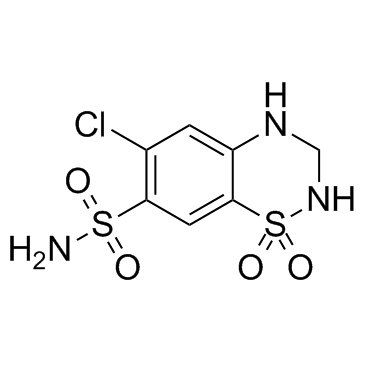
Hydrochlorothiazide structure
|
Common Name | Hydrochlorothiazide | ||
|---|---|---|---|---|
| CAS Number | 58-93-5 | Molecular Weight | 297.739 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 577.0±60.0 °C at 760 mmHg | |
| Molecular Formula | C7H8ClN3O4S2 | Melting Point | 273 °C | |
| MSDS | Chinese USA | Flash Point | 302.7±32.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of HydrochlorothiazideHydrochlorothiazide is a diuretic drug of the thiazide class. Target: OthersHydrochlorothiazide belongs to thiazide class of diuretics. It reduces blood volume by acting on the kidneys to reduce sodium (Na) reabsorption in the distal convoluted tubule. The major site of action in the nephron appears on an electroneutral Na+-Cl? co-transporter by competing for the chloride site on the transporter. By impairing Na transport in the distal convoluted tubule, hydrochlorothiazide induces a natriuresis and concomitant water loss. Thiazides increase the reabsorption of calcium in this segment in a manner unrelated to sodium transport. Additionally, by other mechanisms, Hydrochlorothiazide is believed to lower peripheral vascular resistance [1]. |
| Name | hydrochlorothiazide |
|---|---|
| Synonym | More Synonyms |
| Description | Hydrochlorothiazide is a diuretic drug of the thiazide class. Target: OthersHydrochlorothiazide belongs to thiazide class of diuretics. It reduces blood volume by acting on the kidneys to reduce sodium (Na) reabsorption in the distal convoluted tubule. The major site of action in the nephron appears on an electroneutral Na+-Cl? co-transporter by competing for the chloride site on the transporter. By impairing Na transport in the distal convoluted tubule, hydrochlorothiazide induces a natriuresis and concomitant water loss. Thiazides increase the reabsorption of calcium in this segment in a manner unrelated to sodium transport. Additionally, by other mechanisms, Hydrochlorothiazide is believed to lower peripheral vascular resistance [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 577.0±60.0 °C at 760 mmHg |
| Melting Point | 273 °C |
| Molecular Formula | C7H8ClN3O4S2 |
| Molecular Weight | 297.739 |
| Flash Point | 302.7±32.9 °C |
| Exact Mass | 296.964478 |
| PSA | 135.12000 |
| LogP | -0.07 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.632 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi,T,F |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24-S36/37-S45-S33-S16-S7 |
| RIDADR | UN 1230 3/PG 2 |
| RTECS | DK9100000 |
| HS Code | 2935009090 |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
cIEF for rapid pKa determination of small molecules: a proof of concept.
Eur. J. Pharm. Sci. 63 , 14-21, (2014) A capillary isoelectric focusing (cIEF) method was developed for the determination of the ionization constants (pKa) of small molecules. Two approaches used to decrease the electroosmotic flow (EOF) w... |
|
|
Purification and inhibition studies with anions and sulfonamides of an α-carbonic anhydrase from the Antarctic seal Leptonychotes weddellii.
Bioorg. Med. Chem. 19 , 1847-51, (2011) A high activity α-carbonic anhydrase (CA, EC 4.2.1.1) has been purified from various tissues of the Antarctic seal Leptonychotes weddellii. The new enzyme, denominated lwCA, has a catalytic activity f... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
| HCTZ |
| 6-Chloro-3,4-Dihydro-(2H)-1,2,4-Benzothiadiazine-7-Sulfonamide 1,1-Dioxide |
| 6-Chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide |
| Hydrochlorothiazide |
| Dichlotride |
| MFCD00051765 |
| Aquazide H |
| HCT |
| Esidrix |
| EINECS 200-403-3 |
| Chlorsulfonamidodihydrobenzothiadiazine dioxide |
| 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-3,4-dihydro-, 1,1-dioxide |
| Esidrex |
| HYDRODIURIL |
| ORETIC |
 CAS#:121-30-2
CAS#:121-30-2 CAS#:50-00-0
CAS#:50-00-0 CAS#:58-94-6
CAS#:58-94-6 CAS#:88150-42-9
CAS#:88150-42-9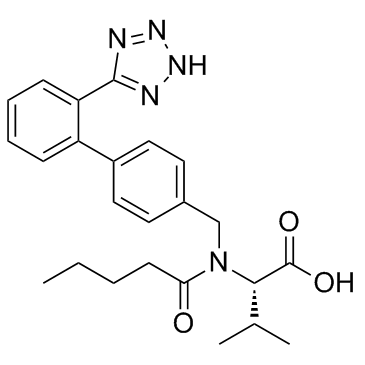 CAS#:137862-53-4
CAS#:137862-53-4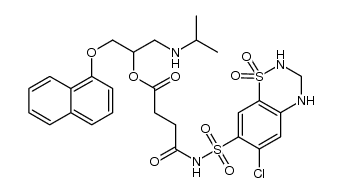 CAS#:1318259-41-4
CAS#:1318259-41-4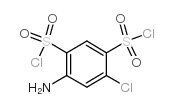 CAS#:671-89-6
CAS#:671-89-6
