H-D-Ser-OMe.HCl
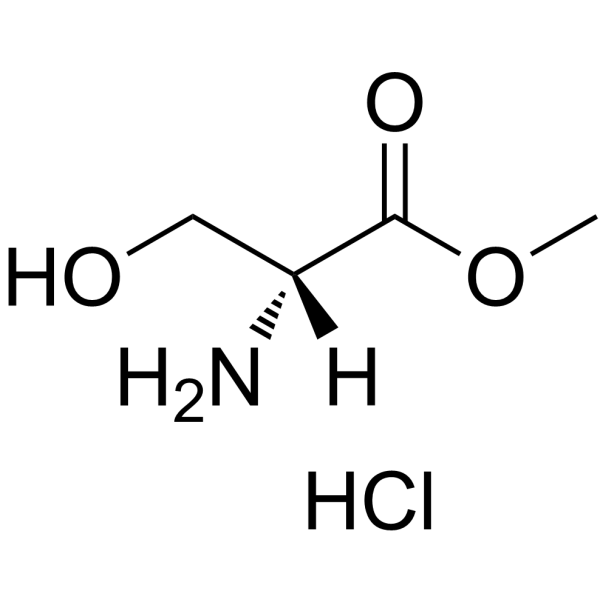
H-D-Ser-OMe.HCl structure
|
Common Name | H-D-Ser-OMe.HCl | ||
|---|---|---|---|---|
| CAS Number | 5874-57-7 | Molecular Weight | 155.580 | |
| Density | 1.62g/cm3 | Boiling Point | 234.7ºC at 760 mmHg | |
| Molecular Formula | C4H10ClNO3 | Melting Point | 163-166 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 95.8ºC | |
Use of H-D-Ser-OMe.HClH-D-Ser-OMe.HCl is a serine derivative[1]. |
| Name | D-Serine methyl ester hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | H-D-Ser-OMe.HCl is a serine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.62g/cm3 |
|---|---|
| Boiling Point | 234.7ºC at 760 mmHg |
| Melting Point | 163-166 °C(lit.) |
| Molecular Formula | C4H10ClNO3 |
| Molecular Weight | 155.580 |
| Flash Point | 95.8ºC |
| Exact Mass | 155.034927 |
| PSA | 72.55000 |
| Index of Refraction | 1.693 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 29225000 |
|
~99% 
H-D-Ser-OMe.HCl CAS#:5874-57-7 |
| Literature: Morieux, Pierre; Salome, Christophe; Park, Ki Duk; Stables, James P.; Kohn, Harold Journal of Medicinal Chemistry, 2010 , vol. 53, # 15 p. 5716 - 5726 |
|
~97% 
H-D-Ser-OMe.HCl CAS#:5874-57-7 |
| Literature: Kim, Hee-Kwon; Park, Kyoung-Joo Jenny Tetrahedron Letters, 2012 , vol. 53, # 13 p. 1668 - 1670 |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Acylated serine derivatives: a unique class of arthropod pheromones of the Australian redback spider, Latrodectus hasselti.
Angew. Chem. Int. Ed. Engl. 49(11) , 2037-40, (2010)
|
|
|
The novel and efficient direct synthesis of N,O-acetal compounds using a hypervalent iodine(III) reagent: an improved synthetic method for a key intermediate of discorhabdins.
Chem. Commun. (Camb.) (13) , 1764-6, (2005) The use of hypervalent iodine(III) reagents allowed us to develop the novel and efficient direct synthesis of N,O-acetal compounds via the oxidative fragmentation reaction of alpha-amino acids or alph... |
|
|
Aziridinium from N,N-dibenzyl serine methyl ester: synthesis of enantiomerically pure beta-amino and alpha,beta-diamino esters.
Org. Lett. 8(10) , 2183-6, (2006) [reaction: see text] Reaction of N,N-dibenzyl-O-methylsulfonyl serine methyl ester with a variety of heteronucleophiles (sodium azide, sodium phthalimide, amines, thiols) and carbanions (sodium malona... |
| L-Serine, methyl ester, hydrochloride (1:1) |
| methyl (2S)-2-amino-3-hydroxypropanoate,hydrochloride |
| DL-Serine, methyl ester, hydrochloride |
| MFCD00066121 |
| Methyl L-serinate hydrochloride (1:1) |
| methyl (2R)-2-amino-3-hydroxypropanoate,hydrochloride |
| methyl DL-serinate hydrochloride |
| Q1YZVO1 &&HCl |
| L-Serine, methyl ester, hydrochloride |
| Serine, methyl ester, hydrochloride (1:1) |
| Methyl serinate hydrochloride |
| Methyl serinate hydrochloride (1:1) |
| DL-serine methylester Hydrochloride |
| D-Serinemethylesterhydrochloride |



 CAS#:108082-29-7
CAS#:108082-29-7 CAS#:55-21-0
CAS#:55-21-0 CAS#:95715-85-8
CAS#:95715-85-8![Methyl N-[(benzyloxy)carbonyl]-D-serinate structure](https://image.chemsrc.com/caspic/244/93204-36-5.png) CAS#:93204-36-5
CAS#:93204-36-5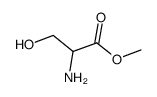 CAS#:2104-89-4
CAS#:2104-89-4
