L-Gulose
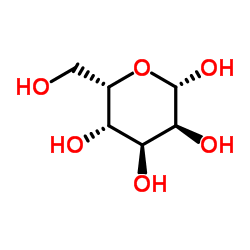
L-Gulose structure
|
Common Name | L-Gulose | ||
|---|---|---|---|---|
| CAS Number | 6027-89-0 | Molecular Weight | 180.156 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 410.8±45.0 °C at 760 mmHg | |
| Molecular Formula | C6H12O6 | Melting Point | 132ºC | |
| MSDS | USA | Flash Point | 202.2±28.7 °C | |
Use of L-GuloseL-Gulose, the putative furanose form of L-sorbosone, is an L-hexose sugar and an intermediate in the biosynthesis of L-Ascorbate (vitamin C)[1]. |
| Name | aldehydo-L-gulose |
|---|---|
| Synonym | More Synonyms |
| Description | L-Gulose, the putative furanose form of L-sorbosone, is an L-hexose sugar and an intermediate in the biosynthesis of L-Ascorbate (vitamin C)[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 410.8±45.0 °C at 760 mmHg |
| Melting Point | 132ºC |
| Molecular Formula | C6H12O6 |
| Molecular Weight | 180.156 |
| Flash Point | 202.2±28.7 °C |
| Exact Mass | 180.063385 |
| PSA | 118.22000 |
| LogP | -1.88 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.635 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 24/25-36/37/39-27-26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2912491000 |
| HS Code | 2912491000 |
|---|---|
| Summary | 2912491000. other aldehyde-alcohols. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Preparation of D-gulose from disaccharide lactitol using microbial and chemical methods.
Biosci. Biotechnol. Biochem. 77(2) , 253-8, (2013) When an M31 strain of Agrobacterium tumefaciens was grown in a mineral salt medium at 30 °C containing 1.0% lactitol as sole carbon source, a keto-sugar was efficiently accumulated in the supernatant.... |
|
|
The role of the gulose-mannose part of bleomycin in activation of iron-molecular oxygen complexes.
Biochem. J. 253(2) , 497-504, (1988) A comparison of the complexing properties of metal ions and O2 activation by bleomycin-A2 (BLM-A2) and deglyco-BLM-A2 is presented. Deglyco-BLM-A2 is obtained from the parent derivative by HF cleavage... |
|
|
Regio- and stereo-selective synthesis of carbohydrate isoxazolidines by 1,3-dipolar cycloaddition of nitrones to 5,6-dideoxy-1,2-O-isopropylidene- alpha-D-xylo-hex-5-enofuranose.
Carbohydr. Res. 226(1) , 49-56, (1992) The synthesis of 2-phenyl-3-aryl and 2-phenyl-3-aroyl derivatives 5-(1,2-O-isopropylidene-alpha-D-xylo-tetrofuranos-4-yl)isoxazolidi ne (3) from nitrones and 5,6-dideoxy-1,2-O-isopropylidene-alpha-D-x... |
| EINECS 227-897-3 |
| MFCD00136022 |
| L-(+)-Gulose |
| L-Gulopyranose |
| Gulopyranose |
| Gulose |
| L-Gulose |
| β-L-gulose |
 CAS#:87-79-6
CAS#:87-79-6 CAS#:17598-82-2
CAS#:17598-82-2 CAS#:5934-56-5
CAS#:5934-56-5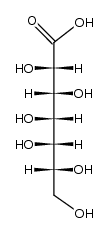 CAS#:35784-84-0
CAS#:35784-84-0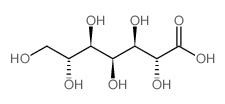 CAS#:882983-01-9
CAS#:882983-01-9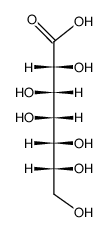 CAS#:689208-16-0
CAS#:689208-16-0
