Bufuralol hydrochloride

Bufuralol hydrochloride structure
|
Common Name | Bufuralol hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 60398-91-6 | Molecular Weight | 297.82000 | |
| Density | N/A | Boiling Point | 393.2ºC at 760 mmHg | |
| Molecular Formula | C16H24ClNO2 | Melting Point | 143-146ºC | |
| MSDS | Chinese USA | Flash Point | 191.6ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Bufuralol hydrochlorideBufuralol hydrochloride (Ro 3-4787 hydrochloride) is a potent non-selective, orally active β-adrenoreceptor antagonist with partial agonist activity. Bufuralol hydrochloride is a CYP2D6 probe substrate[1][2]. |
| Name | (±)-Bufuralol hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Bufuralol hydrochloride (Ro 3-4787 hydrochloride) is a potent non-selective, orally active β-adrenoreceptor antagonist with partial agonist activity. Bufuralol hydrochloride is a CYP2D6 probe substrate[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Bufuralol is widely used in the characterization of CYP2D6 activity, and possesses aromatic rings and a basic nitrogen that are characteristic of CYP2D6 substrates[3]. |
| In Vivo | Bufuralol metabolism mediated by NADPH exhibits biphasic kinetics and is less efficient than that observed in the presence of cumene hydroperoxide (CuOOH) in and monkey intestines, in agreement with the observations in the livers[4]. |
| References |
| Boiling Point | 393.2ºC at 760 mmHg |
|---|---|
| Melting Point | 143-146ºC |
| Molecular Formula | C16H24ClNO2 |
| Molecular Weight | 297.82000 |
| Flash Point | 191.6ºC |
| Exact Mass | 297.15000 |
| PSA | 45.40000 |
| LogP | 4.60960 |
|
Binding of bufuralol, dextromethorphan, and 3,4-methylenedioxymethylamphetamine to wild-type and F120A mutant cytochrome P450 2D6 studied by resonance Raman spectroscopy.
Biochem. Biophys. Res. Commun. 343(3) , 772-9, (2006) Cytochrome P450 2D6 (CYP2D6) is one of the most important drug-metabolizing enzymes in humans. Resonance Raman data, reported for the first time for CYP2D6, show that the CYP2D6 heme is found to be in... |
|
|
The ability of cytochrome P450 2D isoforms to synthesize dopamine in the brain: An in vitro study.
Eur. J. Pharmacol. 626(2-3) , 171-8, (2010) The present study was aimed at determining which rat cytochrome P450 (CYP) isoforms are involved in the hydroxylation of tyramine to dopamine and at determining whether the reaction can take place in ... |
|
|
Predictions of cytochrome P450-mediated drug-drug interactions using cryopreserved human hepatocytes: comparison of plasma and protein-free media incubation conditions.
Drug Metab. Dispos. 40(4) , 706-16, (2012) Cryopreserved human hepatocytes suspended in human plasma (HHSHP) have previously provided accurate CYP3A drug-drug interaction (DDI) predictions from a single IC(50) that captures both reversible and... |
| 1-(7-Ethyl-1-benzofuran-2-yl)-2-[(2-methyl-2-propanyl)amino]ethan ol hydrochloride (1:1) |
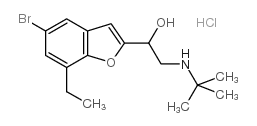 CAS#:137740-36-4
CAS#:137740-36-4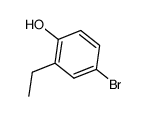 CAS#:18980-21-7
CAS#:18980-21-7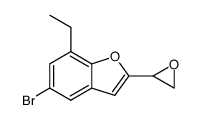 CAS#:69543-78-8
CAS#:69543-78-8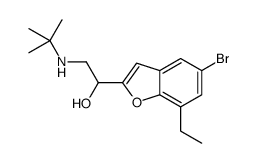 CAS#:85536-86-3
CAS#:85536-86-3 CAS#:57704-12-8
CAS#:57704-12-8 CAS#:137206-65-6
CAS#:137206-65-6