Homopterocarpin
Modify Date: 2024-01-11 18:00:10

Homopterocarpin structure
|
Common Name | Homopterocarpin | ||
|---|---|---|---|---|
| CAS Number | 606-91-7 | Molecular Weight | 284.306 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 395.0±42.0 °C at 760 mmHg | |
| Molecular Formula | C17H16O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 140.4±34.7 °C | |
Use of HomopterocarpinHomopterocarpin is an isoflavonoid that can be isolated from Pterocarpus erinaceus. Homopterocarpin has hepatoprotective and antioxidant properties. Homopterocarpin is a competitive reversible inhibitor of human monoamine oxidase-B with an IC50 and a Ki of 0.72 and 0.21 μM for hMAO-B, respectively. Homopterocarpin can be used for the research of liver injury and oxidative stress[1][2]. |
| Name | Homopterocarpin |
|---|---|
| Synonym | More Synonyms |
| Description | Homopterocarpin is an isoflavonoid that can be isolated from Pterocarpus erinaceus. Homopterocarpin has hepatoprotective and antioxidant properties. Homopterocarpin is a competitive reversible inhibitor of human monoamine oxidase-B with an IC50 and a Ki of 0.72 and 0.21 μM for hMAO-B, respectively. Homopterocarpin can be used for the research of liver injury and oxidative stress[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 395.0±42.0 °C at 760 mmHg |
| Molecular Formula | C17H16O4 |
| Molecular Weight | 284.306 |
| Flash Point | 140.4±34.7 °C |
| Exact Mass | 284.104858 |
| PSA | 36.92000 |
| LogP | 3.14 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.589 |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2932999099 |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 6H-Benzofuro[3,2-c][1]benzopyran, 6a,11a-dihydro-3,9-dimethoxy-, (6aR,11aR)-rel- |
| 6H-Benzofuro[3,2-c][1]benzopyran, 6a,11a-dihydro-3,9-dimethoxy-, (6aR,11aR)- |
| 6H-Benzofuro[3,2-c][1]benzopyran, 6a,11a-dihydro-3,9-dimethoxy-, (6aR-cis)- |
| (6aR,11aR)-3,9-Dimethoxy-6a,11a-dihydro-6H-[1]benzofuro[3,2-c]chromene |
| 6H-Benzofuro(3,2-c)(1)benzopyran, 6a,11a-dihydro-3,9-dimethoxy-, (6aR,11aR)-rel- |
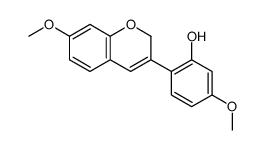 CAS#:3187-50-6
CAS#:3187-50-6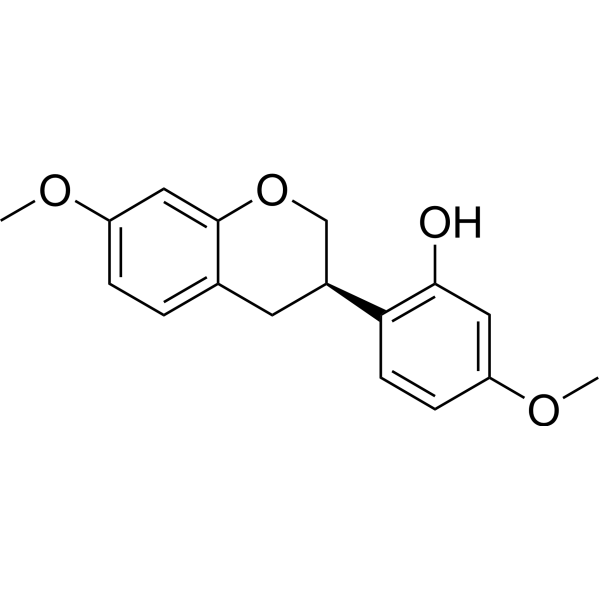 CAS#:60102-29-6
CAS#:60102-29-6