SB742457
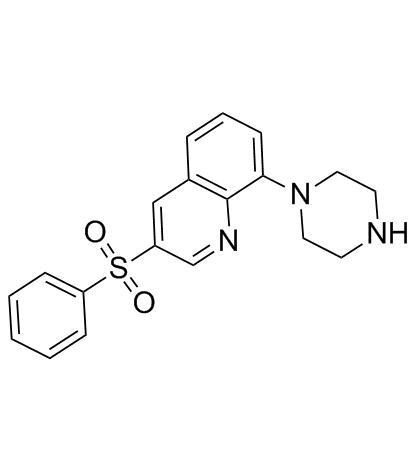
SB742457 structure
|
Common Name | SB742457 | ||
|---|---|---|---|---|
| CAS Number | 607742-69-8 | Molecular Weight | 353.438 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 608.3±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H19N3O2S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 321.7±28.7 °C | |
Use of SB742457SB742457 is a highly selective 5-HT6 receptor antagonist with pKi of 9.63; exhibits >100-fold selectivity over other receptors.IC50 Value: 9.63 (pKi)Target: 5-HT6 ReceptorSB-742457, a 5-HT6 receptor antagonist, which extends into Alzheimer disease (AD) sufferers further highlights the therapeutic promise of this mechanistic approach. Alzheimer's disease is a devastating neurological condition characterized by a progressive decline in cognitive performance accompanied by behavioral and psychological syndromes, such as depression and psychosis. With the subsequent development of selective 5-HT6 receptor antagonists, preclinical studies in rodents and primates have elucidated the function of this receptor subtype in more detail. It is increasingly clear that blockade of 5-HT6 receptors leads to an improvement of cognitive performance in a wide variety of learning and memory paradigms and also results in anxiolytic and antidepressant-like activity. SB-742457 is generally safe and well tolerated and may be efficacious in Alzheimer disease. |
| Name | 3-(benzenesulfonyl)-8-piperazin-1-ylquinoline |
|---|---|
| Synonym | More Synonyms |
| Description | SB742457 is a highly selective 5-HT6 receptor antagonist with pKi of 9.63; exhibits >100-fold selectivity over other receptors.IC50 Value: 9.63 (pKi)Target: 5-HT6 ReceptorSB-742457, a 5-HT6 receptor antagonist, which extends into Alzheimer disease (AD) sufferers further highlights the therapeutic promise of this mechanistic approach. Alzheimer's disease is a devastating neurological condition characterized by a progressive decline in cognitive performance accompanied by behavioral and psychological syndromes, such as depression and psychosis. With the subsequent development of selective 5-HT6 receptor antagonists, preclinical studies in rodents and primates have elucidated the function of this receptor subtype in more detail. It is increasingly clear that blockade of 5-HT6 receptors leads to an improvement of cognitive performance in a wide variety of learning and memory paradigms and also results in anxiolytic and antidepressant-like activity. SB-742457 is generally safe and well tolerated and may be efficacious in Alzheimer disease. |
|---|---|
| Related Catalog | |
| References |
[6]. SB-742457 |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 608.3±45.0 °C at 760 mmHg |
| Molecular Formula | C19H19N3O2S |
| Molecular Weight | 353.438 |
| Flash Point | 321.7±28.7 °C |
| Exact Mass | 353.119812 |
| PSA | 70.68000 |
| LogP | 2.10 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.649 |
| Storage condition | -20℃ |
|
~73% 
SB742457 CAS#:607742-69-8 |
| Literature: GLAXO GROUP LIMITED Patent: WO2005/26125 A1, 2005 ; Location in patent: Page/Page column 21-22 ; WO 2005/026125 A1 |
|
~84% 
SB742457 CAS#:607742-69-8 |
| Literature: GLAXO GROUP LIMITED Patent: WO2007/39238 A1, 2007 ; Location in patent: Page/Page column 14-16 ; |
|
~69% 
SB742457 CAS#:607742-69-8 |
| Literature: Emmett, Edward J.; Hayter, Barry R.; Willis, Michael C. Angewandte Chemie - International Edition, 2013 , vol. 52, # 48 p. 12679 - 12683 Angew. Chem., 2013 , vol. 125, # 48 p. 12911 - 12915,5 |
|
~% 
SB742457 CAS#:607742-69-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 57, # 9 p. 3884 - 3890 |
|
~% 
SB742457 CAS#:607742-69-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 57, # 9 p. 3884 - 3890 |
|
~% 
SB742457 CAS#:607742-69-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 57, # 9 p. 3884 - 3890 |
|
~% 
SB742457 CAS#:607742-69-8 |
| Literature: Journal of Medicinal Chemistry, , vol. 57, # 9 p. 3884 - 3890 |
| SB-742,457 |
| Quinoline,3-(phenylsulfonyl)-8-(1-piperazinyl) |
| RVT-101 |
| GSK-742457 |
| 3-(phenylsulfonyl)-8-(piperazin-1-yl)quinoline |
| Intepirdine |
| Quinoline, 3-(phenylsulfonyl)-8-(1-piperazinyl)- |
| UNII-2IOB2M82HY |
| SB-742457 |
| 3-(Phenylsulfonyl)-8-(1-piperazinyl)quinoline |
| SB742457 |

![tert-butyl 4-[3-(phenylsulfonyl)quinolin-8-yl]piperazine-1-carboxylate structure](https://image.chemsrc.com/caspic/164/607743-10-2.png)