Geraniin
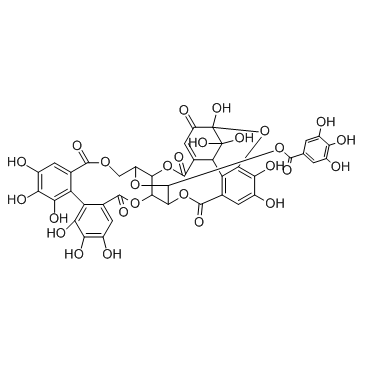
Geraniin structure
|
Common Name | Geraniin | ||
|---|---|---|---|---|
| CAS Number | 60976-49-0 | Molecular Weight | 952.645 | |
| Density | 2.3±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C41H28O27 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of GeraniinGeraniin is a TNF-α releasing inhibitor with numerous activities including anticancer, anti-inflammatory, and anti-hyperglycemic activities, with an IC50 of 43 μM. |
| Name | b-D-Glucopyranose |
|---|---|
| Synonym | More Synonyms |
| Description | Geraniin is a TNF-α releasing inhibitor with numerous activities including anticancer, anti-inflammatory, and anti-hyperglycemic activities, with an IC50 of 43 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 43 μM (TNF-α)[1]. |
| In Vitro | The IC50 value of TNF-α release inhibition is 43 μM for Geraniin[1]. Geraniin has long been used as a medicinal herb and possesses numerous activities including anticancer, anti-inflammatory, and anti-hyperglycemic activities. Geraniin significantly decreases the viability of OVCAR3 and SKOV3 cells in a concentration-dependent fashion. The IC50 value for Geraniin treatment is 34.5±2.8 μM in OVCAR3 cells and 23.6±1.9 μM in SKOV3 cells. However, Geraniin up to the maximal concentration used (80 μM) has no significant impact on the viability of normal human ovarian surface epithelial cells. Treatment with 10 and 40 μM of Geraniin for 48 h causes a significant increase in apoptosis (16.8±1.2% and 22.6±1.4%, respectively), compared with control OVCAR3 cells (3.9±1.1%). Similar results are observed in SKOV3 cells[2]. |
| In Vivo | Treatment with Geraniin prior to application of okadaic acid delays development of tumors compared with control group, reduces the percentage of tumor bearing mice from 80.0% to 40.0%, and reduces the average numbers of tumor per mouse from 3.8 to 1.1 in week 20. It is also showed that oral administration of Geraniin to rats (50 mg/kg/d or 100 mg/kg/d) inhibit the elevation of serum total cholesterol, lipid peroxide, free fatty acid, triglyceride, glutamic oxaloacetic transaminase and glutamic pyruvic transaminase induced by treatment with peroxidized oil[1]. |
| Cell Assay | Human ovarian cancer cell lines OVCAR3 and SKOV3 are used. Cells are exposed to different concentrations (5, 10, 20, 40, and 80 μM) of Geraniin for 48 h and examined for viability, apoptosis, and gene expression. The concentration range is selected based on previous studies[2]. |
| References |
| Density | 2.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C41H28O27 |
| Molecular Weight | 952.645 |
| Exact Mass | 952.081787 |
| PSA | 450.25000 |
| LogP | 3.41 |
| Index of Refraction | 1.948 |
| HS Code | 2932999099 |
|---|
|
~% 
Geraniin CAS#:60976-49-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 34, # 10 p. 4075 - 4082 |
|
~% 
Geraniin CAS#:60976-49-0 |
| Literature: Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), , p. 369 - 376 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| b-d-glucopyranose |
| Benzoic acid, 3,4,5-trihydroxy-, (3R,4aR,11R,11aS,25R,25aR,29S)-2,3,4,4a,9,11a,13,22,24,25,25a,27-dodecahydro-3,4,4,6,7,15,16,17,18,19,20-undecahydroxy-2,9,13,22,27-pentaoxo-3,5-epoxy-25,11-(epoxymethano)-11H-dibenzo[h,j]dibenzo[7,8:9,10][1,5]dioxacycloundecino[3,2-b][1,6]dioxacyclododecin-29-yl ester |
| Geraniin |
| (1R,7R,8S,26R,28S,29R,38R)-1,13,14,15,18,19,20,34,35,39,39-Undecahydroxy-2,5,10,23,31-pentaoxo-6,9,24,27,30,40-hexaoxaoctacyclo[34.3.1.0.0.0.0.0.0]tetraconta-3,1 ; 1,13,15,17,19,21,32,34,36-decaen-28-yl 3,4,5-trihydroxybenzoate |
| (1R,7R,8S,26R,28S,29R,38R)-1,13,14,15,18,19,20,34,35,39,39-Undecahydroxy-2,5,10,23,31-pentaoxo-6,9,24,27,30,40-hexaoxaoctacyclo[34.3.1.0.0.0.0.0.0]tetraconta-3,11,13,15,17,19,21,32,34,36-decaen-28-yl 3,4,5-trihydroxybenzoate |
 CAS#:1707-75-1
CAS#:1707-75-1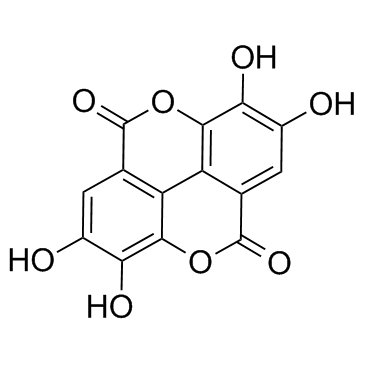 CAS#:476-66-4
CAS#:476-66-4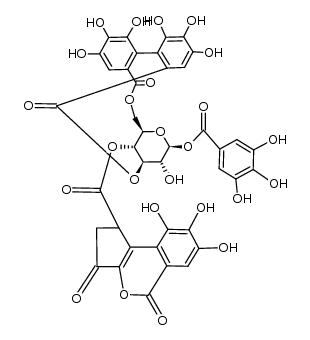 CAS#:132185-49-0
CAS#:132185-49-0 CAS#:100227-56-3
CAS#:100227-56-3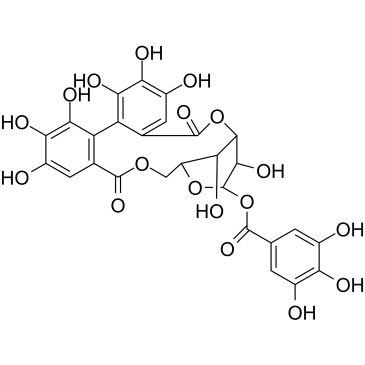 CAS#:23094-69-1
CAS#:23094-69-1 CAS#:125516-10-1
CAS#:125516-10-1
