4-quinolone
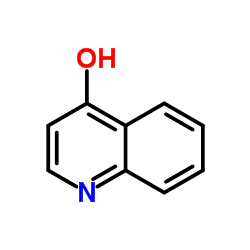
4-quinolone structure
|
Common Name | 4-quinolone | ||
|---|---|---|---|---|
| CAS Number | 611-36-9 | Molecular Weight | 145.158 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 313.0±15.0 °C at 760 mmHg | |
| Molecular Formula | C9H7NO | Melting Point | 200-202 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 143.1±20.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 4-quinolone4-Quinolone (Kynurine) is a quinoline derivative. Kynurine pathway modulates tryptophan metabolism and involves in neuroprotective effect. Kynurine promotes tumor cell survival and motility by suppressing antitumor immune[1][2]. |
| Name | quinolin-4-ol |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Quinolone (Kynurine) is a quinoline derivative. Kynurine pathway modulates tryptophan metabolism and involves in neuroprotective effect. Kynurine promotes tumor cell survival and motility by suppressing antitumor immune[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | 4-Quinolone (Kynurine) promotes tumor cell survival and motility by suppressing antitumor immune responses through AhR in an autocrine/paracrine fashion. This system is particularly active in human brain tumors where the activation of AhR enhances the expression of TDO producing more kynurine and kynurinic acid. In the healthy human brain, very low levels of TDO exist, whereas, in human brain tumors, the TDO protein levels increase with malignancy[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 313.0±15.0 °C at 760 mmHg |
| Melting Point | 200-202 °C(lit.) |
| Molecular Formula | C9H7NO |
| Molecular Weight | 145.158 |
| Flash Point | 143.1±20.4 °C |
| Exact Mass | 145.052765 |
| PSA | 33.12000 |
| LogP | 2.45 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.691 |
CHEMICAL IDENTIFICATION
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | VC4070000 |
| HS Code | 2933499090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synergistic antidepressant-like effect of ferulic acid in combination with piperine: involvement of monoaminergic system.
Metab. Brain Dis. 30 , 1505-14, (2015) The lifetime prevalence rate for major depressive disorder (MDD) is approximately 17 % for most developed countries around the world. Dietary polyphenols are currently used as an adjuvant therapy to a... |
|
|
Evolution from a natural flavones nucleus to obtain 2-(4-Propoxyphenyl)quinoline derivatives as potent inhibitors of the S. aureus NorA efflux pump.
J. Med. Chem. 54 , 5722-36, (2011) Overexpression of efflux pumps is an important mechanism by which bacteria evade the effects of substrate antimicrobial agents. Inhibition of such pumps is a promising strategy to circumvent this resi... |
|
|
Antioxidant properties of 4-quinolones and structurally related flavones.
Bioorg. Med. Chem. 20 , 809-18, (2012) Neurodegenerative disorders are frequently associated with increased oxidative damage to the brain as a result of free radicals produced by cellular respiration. The onset and progression of neurodege... |
| 4-Quinolinol |
| Chinolin-4-ol |
| p-hydroxyquinoline |
| 4-quinolone |
| 4(1H)-QUINOLINONE |
| Chinolin-4(1H)-on |
| Quinolin-4(1H)-one |
| quinolin-4-one |
| 1,4-Dihydroquinoline-4-one |
| HYDROXYQUINOLINE,4 |
| 4-hydroxyquinoline acid |
| KYNURINE |
| 4-Hydroxyquinoline |
| 4-oxo-1,4-dihydroquinoline |
| 4-hydroxy-quinolin |
| EINECS 210-268-2 |
| quinolin-4-ol |
| quinoline-4-ol |
| MFCD00006777 |
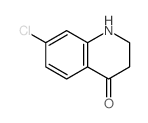 CAS#:21617-15-2
CAS#:21617-15-2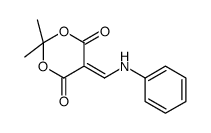 CAS#:15568-92-0
CAS#:15568-92-0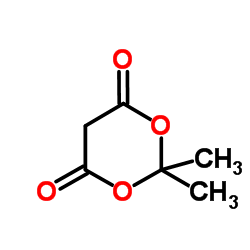 CAS#:2033-24-1
CAS#:2033-24-1 CAS#:611-35-8
CAS#:611-35-8 CAS#:1613-37-2
CAS#:1613-37-2 CAS#:79-36-7
CAS#:79-36-7 CAS#:23981-80-8
CAS#:23981-80-8 CAS#:54-12-6
CAS#:54-12-6 CAS#:67-56-1
CAS#:67-56-1 CAS#:39061-97-7
CAS#:39061-97-7 CAS#:607-31-8
CAS#:607-31-8 CAS#:83-54-5
CAS#:83-54-5![2-ethyl-1-(2-methylpropyl)imidazo[4,5-c]quinolin-4-amine structure](https://image.chemsrc.com/caspic/245/149876-20-0.png) CAS#:149876-20-0
CAS#:149876-20-0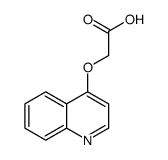 CAS#:146363-08-8
CAS#:146363-08-8![N-[1-(2-methylpropyl)imidazo[4,5-c]quinolin-4-yl]benzamide structure](https://image.chemsrc.com/caspic/101/144660-62-8.png) CAS#:144660-62-8
CAS#:144660-62-8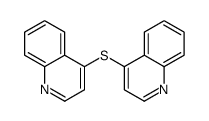 CAS#:14095-26-2
CAS#:14095-26-2 CAS#:42606-33-7
CAS#:42606-33-7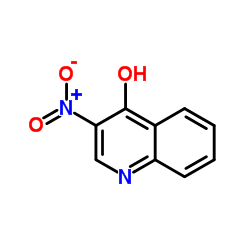 CAS#:50332-66-6
CAS#:50332-66-6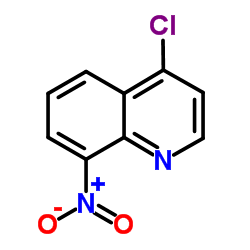 CAS#:23833-99-0
CAS#:23833-99-0
