AZD1080
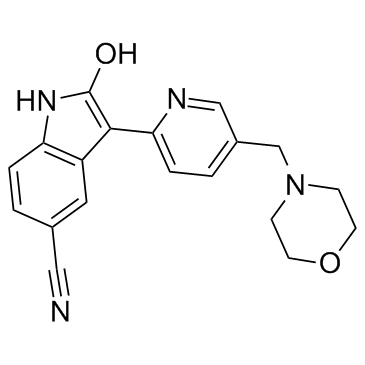
AZD1080 structure
|
Common Name | AZD1080 | ||
|---|---|---|---|---|
| CAS Number | 612487-72-6 | Molecular Weight | 334.372 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 594.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C19H18N4O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 313.0±30.1 °C | |
Use of AZD1080AZD1080 is a potent and selective GSK3 inhibitor. AZD1080 inhibits recombinant human GSK3α and GSK3β with pKi (IC50) of 8.2 (6.9 nM) and 7.5 (31 nM), respectively. |
| Name | 3-[5-(morpholin-4-ylmethyl)-1H-pyridin-2-ylidene]-2-oxo-1H-indole-5-carbonitrile |
|---|---|
| Synonym | More Synonyms |
| Description | AZD1080 is a potent and selective GSK3 inhibitor. AZD1080 inhibits recombinant human GSK3α and GSK3β with pKi (IC50) of 8.2 (6.9 nM) and 7.5 (31 nM), respectively. |
|---|---|
| Related Catalog | |
| Target |
GSK-3α:8.2 (pKi) GSK-3β:7.5 (pKi) cdk5:6.4 (pKi) cdk2:5.9 (pKi) cdk1:5.7 (pKi) |
| In Vitro | AZD1080 shows selectivity against cdk2 (pKi=5.9; 1150 nM; 37-fold), cdk5 (pKi=6.4; 429 nM; 14-fold), cdk1 (pKi=5.7; 1980 nM; 64-fold) and Erk2 (pKi< 5; >10 μM; >323-fold). AZD1080 (at 10 μM) is also evaluated for pan-kinase selectivity and showed good overall selectivity versus 23 kinases, as well as against 65 different receptors, enzymes and ion channels in MDS Pharma screen (< 50% effect at 10 μM AZD1080). Concentration-dependent inhibition of tau phosphorylation is observed for AZD1080 (IC50=324 nM) and the non-selective reference GSK3 inhibitor LiCl (IC50=1.5 mM) indicating that AZD1080 is several orders of magnitude more potent than LiCl[1]. |
| In Vivo | The pharmacokinetic analysis in blood after oral administration revealed that AZD1080 has a good oral bioavailability in rats (15-24%) with a half-life of 7.1 h, making AZD1080 attractive for further in vivo testing. The subchronic (3 days) oral treatment with AZD1080 at 4 or 15 μmol/kg significantly blocked the MK-801-induced memory deficit (AZD1080 vs. MK-801, p<0.05 at 4 μmol/kg and p<0.01 at 15 μmol/kg) in mice, raising the hypothesis that longer treatment may be required to prime the synapses to function effectively[1]. |
| Kinase Assay | The GSK3β, Cdk2, and Cdk5 Ki’s are determined using scintillation proximity assays and kinetic analyses. The GSK3α assay is performed for the GSK3β assay. The KM value of ATP used to calculate the Ki value for GSKα is 10 μM. Inhibition of Cdk1 is performed. The KM value of ATP used to calculate the Ki value is 51 μM. Erk2 activity is determined using an Ser/Thr kinase SPA kit, p42 MAPK kinase (20 U/well), and biotinylated MBP. The KM value of ATP used to calculate the Ki value is 71 μM[1]. |
| Animal Admin | Mice[1] A total of 161 male C57BL/6 mice, 8-12 weeks of age, are used. The animals are kept in conventional housing (3-5 mice per cage) and fed standard rodent chew and tap water ad libitum. Typically 9-12 mice are included in each experimental group and 2-4 mice in the satellite groups (for determination of compound exposure in plasma and brain, see below). AZD1080 (4.0 or 15 μmol/kg) or vehicle (water with 0.5% ascorbic acid, 0.01% EDTA, pH 2.0) is administered by oral gavage (10 mL/kg) acutely or subchronically (twice daily) for 3 days. The training trial is performed at 1.5, 3, or 5 h after final administration with AZD1080. To disrupt learning, the mice received subcutaneous administration of MK-801 (0.1 or 0.15 mg/kg; (+)-MK.801 hydrogen maleate) or vehicle (saline) 30 min before the training trial. Rats[1] A total of 71 adult male Sprague-Dawley rats (250-300 g) are used. The rats receive an acute dose of AZD1080 (1, 3 or 10 μmol/kg) or vehicle (water with 0.5% ascorbic acid, 0.01% EDTA, pH 2.0) via oral gavage (dosing volume 5 mL/kg). At 1, 2, 3, 6, or 24 h after administration the rats are anesthetized and blood, from abdominal aorta, is sampled in heparin micro tainer tubes. Peripheral blood mononuclear cells (PBMC) are isolated from the blood samples. Separate blood samples are obtained for plasma processing and subsequent bioanalysis. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 594.0±50.0 °C at 760 mmHg |
| Molecular Formula | C19H18N4O2 |
| Molecular Weight | 334.372 |
| Flash Point | 313.0±30.1 °C |
| Exact Mass | 334.142975 |
| PSA | 85.17000 |
| LogP | 1.36 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.716 |
| InChIKey | BLTVBQXJFVRPFK-UHFFFAOYSA-N |
| SMILES | N#Cc1ccc2[nH]c(O)c(-c3ccc(CN4CCOCC4)cn3)c2c1 |
| Storage condition | -20°C |
|
~73% 
AZD1080 CAS#:612487-72-6 |
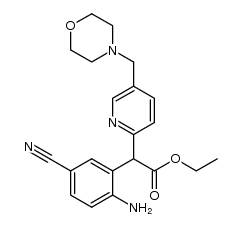
| Literature: ASTRAZENECA AB Patent: WO2007/89193 A1, 2007 ; Location in patent: Page/Page column 23-24 ; WO 2007/089193 A1 |
|
~81% 
AZD1080 CAS#:612487-72-6 |
| Literature: ASTRAZENECA AB Patent: WO2008/130312 A1, 2008 ; Location in patent: Page/Page column 37 ; WO 2008/130312 A1 |
|
~48% 
AZD1080 CAS#:612487-72-6 |
| Literature: ASTRAZENECA AB Patent: WO2008/130312 A1, 2008 ; Location in patent: Page/Page column 36 ; WO 2008/130312 A1 |
|
~6% 
AZD1080 CAS#:612487-72-6 |
| Literature: ASTRAZENECA AB Patent: WO2003/82853 A1, 2003 ; Location in patent: Page/Page column 59 ; WO 03/082853 A1 |
|
~% 
AZD1080 CAS#:612487-72-6 |
| Literature: Ryberg, Per Organic Process Research and Development, 2008 , vol. 12, # 3 p. 540 - 543 |
| 2-Hydroxy-3-[5-(4-morpholinylmethyl)-2-pyridinyl]-1H-indole-5-carbonitrile |
| 2-hydroxy-3-[5-(morpholin-4-ylmethyl)pyridin-2-yl]-1H-indole-5-carbonitrile |
| 1H-INDOLE-5-CARBONITRILE,2-HYDROXY-3-[5-(4-MORPHOLINYLMETHYL)-2-PYRIDINYL] |
| Kinome_2945 |
| AZD1080 |
![5-bromo-3-[5-(morpholin-4-ylmethyl)-1H-pyridin-2-ylidene]-1H-indol-2-one structure](https://image.chemsrc.com/caspic/214/612488-09-2.png)