lissamine rhodamine b sulfonyl chloride
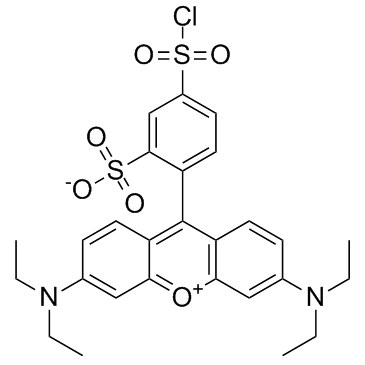
lissamine rhodamine b sulfonyl chloride structure
|
Common Name | lissamine rhodamine b sulfonyl chloride | ||
|---|---|---|---|---|
| CAS Number | 62796-29-6 | Molecular Weight | 577.11200 | |
| Density | 1.1576 (rough estimate) | Boiling Point | N/A | |
| Molecular Formula | C27H29ClN2O6S2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of lissamine rhodamine b sulfonyl chlorideSulforhodamine B acid chloride is a fluorescent protein label forming stable conjugates. |
| Name | lissamine rhodamine b sulfonyl chloride |
|---|---|
| Synonym | More Synonyms |
| Description | Sulforhodamine B acid chloride is a fluorescent protein label forming stable conjugates. |
|---|---|
| Related Catalog | |
| In Vitro | Sulforhodamine B (SRB) is often used as a membrane-impermeable polar tracer or used for cell density determination via determination of cellular proteins (cytotoxicity assay). The SRB assay has been used to inexpensively conduct various screening assays to investigate cytotoxicity in cell based studies. This method relies on the property of SRB, which binds stoichiometrically to proteins under mild acidic conditions and then can be extracted using basic conditions; thus, the amount of bound dye can be used as a proxy for cell mass, which can then be extrapolated to measure cell proliferation. The protocol can be divided into four main steps: preparation of treatment, incubation of cells with treatment of choice, cell fixation and SRB staining, and absorbance measurement. This assay is limited to manual or semiautomatic screening, and can be used in an efficient and sensitive manner to test chemotherapeutic drugs or small molecules in adherent cells. It also has applications in evaluating the effects of gene expression modulation (knockdown, gene expression upregulation), as well as to study the effects of miRNA replacement on cell proliferation[1]. |
| Cell Assay | Gently add 25 μL (96-well format) or 5 μL (384-well format) cold 50% (wt/vol) TCA to each well directly to medium supernatant, and incubate the plates at 4 °C for 1 h. Mixing is not required, as this could lead to some cells detaching from the bottom of the well. Wash the plates four times by submerging the plate in a tub with slow-running tap water and remove excess water by gently tapping the plate into a paper towel. After the last wash allow the plate to air-dry at room temperature. Add 50 μL (96-well format) or 20 μL (384-well format) of 0.04% (wt/vol) SRB solution to each well. Leave at room temperature for 1 h and then quickly rinse the plates four times with 1% (vol/vol) acetic acid (200 μL for 96-well format or 30 μL for 384-well format) to remove unbound dye. Allow the plate to air-dry at room temperature[1]. |
| References |
| Density | 1.1576 (rough estimate) |
|---|---|
| Molecular Formula | C27H29ClN2O6S2 |
| Molecular Weight | 577.11200 |
| Exact Mass | 576.11600 |
| PSA | 127.49000 |
| LogP | 6.85600 |
| Index of Refraction | 1.6100 (estimate) |
| Storage condition | −20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2921199090 |
|
~88% 
lissamine rhoda... CAS#:62796-29-6 |
| Literature: Yang, Hua; Vasudevan, Sundar; Oriakhi, Christopher O.; Shields, Jay; Carter, Rich G. Synthesis, 2008 , # 6 p. 957 - 961 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| HS Code | 2921199090 |
|---|---|
| Summary | 2921199090 other acyclic monoamines and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
A novel conjugable translocator protein ligand labeled with a fluorescence dye for in vitro imaging.
Bioconjug. Chem. 18(4) , 1118-22, (2007) A conjugable analogue of the benzodiazepine 4' '-chlorodiazepam (Ro5-4864), C6Ro5-4864 was synthesized to probe the binding sites of translocator protein (18 kDa; TSPO), previously known as the periph... |
|
|
Local immunity to Bacteroides gingivalis in periodontal disease.
J. Dent. Res. 59 , 1750-6, (1980) The histopathologic status of gingival tissue was assessed according to the criteria of Page and Schroeder.(5) Rhodamine-labeled bacteria were used to assess the antigenic specificity of plasma cells ... |
|
|
Psoralen conjugates for visualization of genomic interstrand cross-links localized by laser photoactivation.
Bioconjug. Chem. 18(2) , 431-7, (2007) DNA interstrand cross-links are formed by chemotherapy drugs as well as by products of normal oxidative metabolism. Despite their importance, the pathways of cross-link metabolism are poorly understoo... |
| MFCD00042001 |
| 5-chlorosulfonyl-2-[3-(diethylamino)-6-diethylazaniumylidenexanthen-9-yl]benzenesulfonate |
| EINECS 263-735-8 |
| Sulforhodamine B acid chloride |
 CAS#:1244034-02-3
CAS#:1244034-02-3