(S)-Cbz-Phenylalaninol
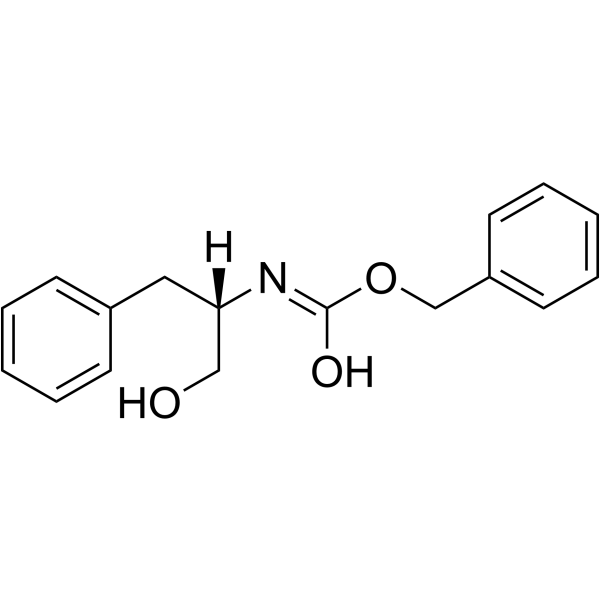
(S)-Cbz-Phenylalaninol structure
|
Common Name | (S)-Cbz-Phenylalaninol | ||
|---|---|---|---|---|
| CAS Number | 6372-14-1 | Molecular Weight | 285.338 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 489.0±45.0 °C at 760 mmHg | |
| Molecular Formula | C17H19NO3 | Melting Point | 90-94ºC | |
| MSDS | Chinese USA | Flash Point | 249.6±28.7 °C | |
Use of (S)-Cbz-PhenylalaninolZ-Phenylalaninol is an alanine derivative[1]. |
| Name | (S)-Cbz-Phenylalaninol |
|---|---|
| Synonym | More Synonyms |
| Description | Z-Phenylalaninol is an alanine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 489.0±45.0 °C at 760 mmHg |
| Melting Point | 90-94ºC |
| Molecular Formula | C17H19NO3 |
| Molecular Weight | 285.338 |
| Flash Point | 249.6±28.7 °C |
| Exact Mass | 285.136505 |
| PSA | 58.56000 |
| LogP | 3.25 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.583 |
| Storage condition | 2~8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | T+ |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924299090 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Preparation of Aminoalkyl Chlorohydrin Hydrochlorides: Key Building Blocks for Hydroxyethylamine-Based HIV Protease Inhibitors.
J. Org. Chem. 61 , 3635, (1996) Enantiomerically pure N,N-dibenzyl-alpha-amino aldehydes reacted with (chloromethyl)lithium, generated in situ from bromochloromethane and lithium metal, to give predominantly erythro aminoalkyl epoxi... |
|
|
Stereoselective Synthesis of HIV-1 Protease Inhibitor, DMP 323.
J. Org. Chem. 61 , 444, (1996) DMP 323, a potent HIV-1 protease inhibitor, has been synthesized by an efficient stereoselective process, amenable to large scale preparations. The core C(2) symmetric diol was synthesized by a stereo... |
|
|
Cyclic HIV protease inhibitors: synthesis, conformational analysis, P2/P2' structure-activity relationship, and molecular recognition of cyclic ureas.
J. Med. Chem. 39 , 3514, (1996) High-resolution X-ray structures of the complexes of HIV-1 protease (HIV-1PR) with peptidomimetic inhibitors reveal the presence of a structural water molecule which is hydrogen bonded to both the mob... |
| Benzyl [(2S)-1-hydroxy-3-phenylpropan-2-yl]carbamate |
| Carbamic acid, N-[(1S)-2-hydroxy-1-(phenylmethyl)ethyl]-, phenylmethyl ester |
| benzyl N-[(2S)-1-hydroxy-3-phenylpropan-2-yl]carbamate |
| Benzyl [(2S)-1-hydroxy-3-phenyl-2-propanyl]carbamate |
| Benzyl (1-hydroxy-3-phenylpropan-2-yl)carbamate |
| Z-L-phenylalaninol;; (S)-2-(Z-amino)-3-phenyl-1-propanol |
| MFCD00191138 |
| Z-Phenylalaninol |
| Z-Phe-ol |
 CAS#:1161-13-3
CAS#:1161-13-3 CAS#:13139-17-8
CAS#:13139-17-8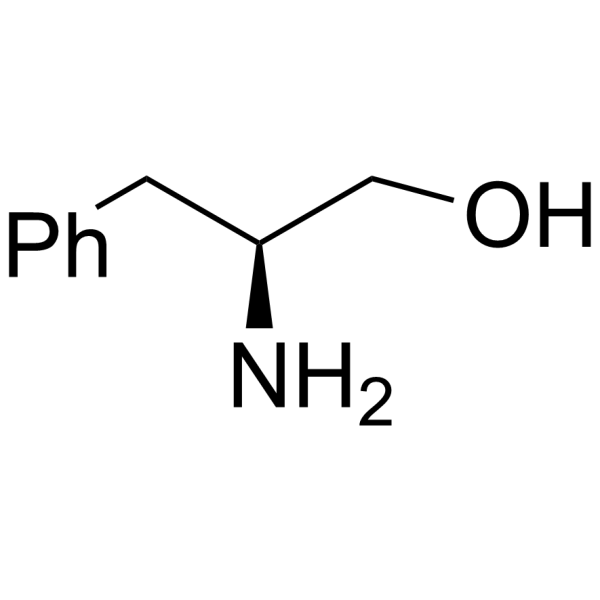 CAS#:3182-95-4
CAS#:3182-95-4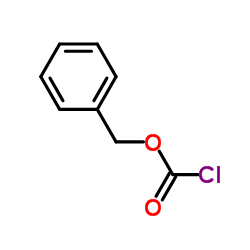 CAS#:501-53-1
CAS#:501-53-1 CAS#:35909-92-3
CAS#:35909-92-3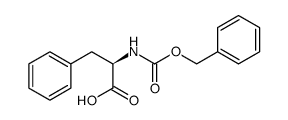 CAS#:28709-70-8
CAS#:28709-70-8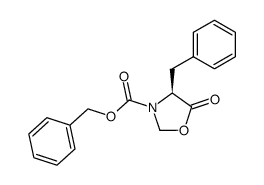 CAS#:55740-07-3
CAS#:55740-07-3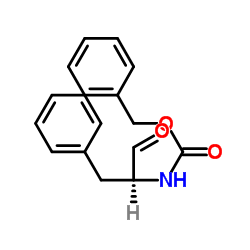 CAS#:59830-60-3
CAS#:59830-60-3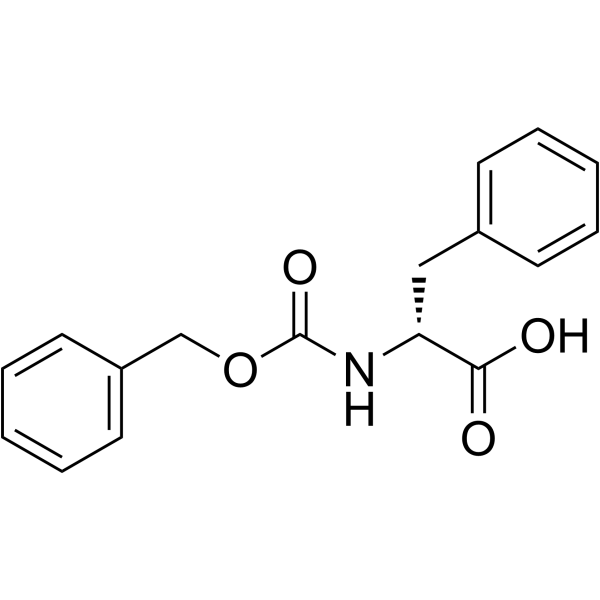 CAS#:2448-45-5
CAS#:2448-45-5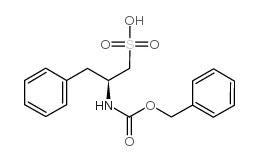 CAS#:856570-20-2
CAS#:856570-20-2 CAS#:63-91-2
CAS#:63-91-2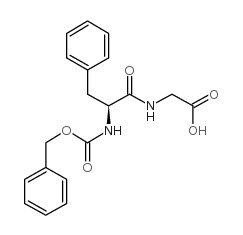 CAS#:13122-99-1
CAS#:13122-99-1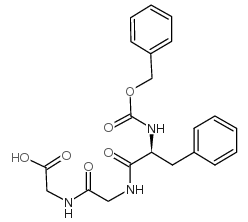 CAS#:37700-64-4
CAS#:37700-64-4
