Practolol
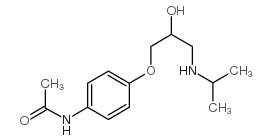
Practolol structure
|
Common Name | Practolol | ||
|---|---|---|---|---|
| CAS Number | 6673-35-4 | Molecular Weight | 266.33600 | |
| Density | 1.0807 (rough estimate) | Boiling Point | 409.54°C (rough estimate) | |
| Molecular Formula | C14H22N2O3 | Melting Point | 134-136° (BuOAc) | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of PractololPractolol is a potent and selective β1-adrenergic receptor antagonist. Practolol can be used for the research of cardiac arrhythmias[1][2][3]. |
| Name | practolol |
|---|---|
| Synonym | More Synonyms |
| Description | Practolol is a potent and selective β1-adrenergic receptor antagonist. Practolol can be used for the research of cardiac arrhythmias[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
β1-adrenergic receptor[1] |
| In Vitro | Practolol (10 μM; 30 min) preferentially antagonizes the relaxant effects produced by R0363 in spontaneously contracted tracheal preparations from the guinea-pig[1]. Practolol (10 nM-100 μM) blocks the progesterone production induced by (-)epinephrine in a dose-dependent manner in granulosa cells[2]. |
| In Vivo | Practolol (0.5 mg/kg; i.v.) decreases heart rate, left ventricular dP/dt max, myocardial blood flow and cardiac output in intact close-chest dogs[3]. Practolol (0.5 mg/kg; i.v.) decreases normal myocardial blood flow but flow in the ischaemic area remains unchanged after coronary artery ligation[3]. |
| References |
| Density | 1.0807 (rough estimate) |
|---|---|
| Boiling Point | 409.54°C (rough estimate) |
| Melting Point | 134-136° (BuOAc) |
| Molecular Formula | C14H22N2O3 |
| Molecular Weight | 266.33600 |
| Exact Mass | 266.16300 |
| PSA | 70.59000 |
| LogP | 1.84660 |
| Index of Refraction | 1.5110 (estimate) |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | AE4222000 |
| HS Code | 2924299090 |
|
~% 
Practolol CAS#:6673-35-4 |
| Literature: Tetrahedron Asymmetry, , vol. 11, # 14 p. 2885 - 2898 |
|
~% 
Practolol CAS#:6673-35-4 |
| Literature: Tetrahedron Asymmetry, , vol. 11, # 14 p. 2885 - 2898 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Large-Scale Prediction of Drug Targets Based on Local and Global Consistency of Chemical-Chemical Networks.
Comb. Chem. High Throughput Screen 19 , 121-8, (2016) It is crucial to identify the molecular targets of a compound during the course of the new drug discovery and drug development. Due to the complexity of biological systems, finding drug targets by bio... |
| N-[4-[2-hydroxy-3-(propan-2-ylamino)propoxy]phenyl]acetamide |
| rac Practolol |

![N-[4-[2-Hydroxy-3-[(1-methylethyl)amino]propoxy]phenyl]-N-methylacetamide structure](https://image.chemsrc.com/caspic/114/57494-85-6.png) CAS#:57494-85-6
CAS#:57494-85-6