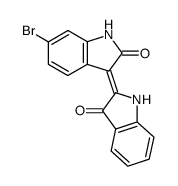
BIO
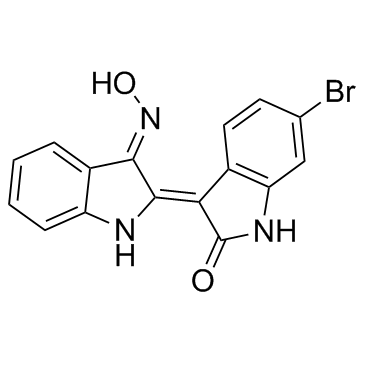
BIO structure
|
Common Name | BIO | ||
|---|---|---|---|---|
| CAS Number | 667463-62-9 | Molecular Weight | 356.173 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 554.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C16H10BrN3O2 | Melting Point | 300°C(lit.) | |
| MSDS | USA | Flash Point | 289.0±30.1 °C | |
Use of BIOGSK 3 Inhibitor IX (6-Bromoindirubin-3'-oxime; BIO) is a potent, selective, reversible and ATP-competitive inhibitor of GSK-3α/β and CDK1-cyclinB complex with IC50s of 5 nM/320 nM/80 nM for (GSK-3α/β)/CDK1/CDK5, respectively. |
| Name | BIO |
|---|---|
| Synonym | More Synonyms |
| Description | GSK 3 Inhibitor IX (6-Bromoindirubin-3'-oxime; BIO) is a potent, selective, reversible and ATP-competitive inhibitor of GSK-3α/β and CDK1-cyclinB complex with IC50s of 5 nM/320 nM/80 nM for (GSK-3α/β)/CDK1/CDK5, respectively. |
|---|---|
| Related Catalog | |
| Target |
GSK-3α:5 nM (IC50) GSK-3β:5 nM (IC50) CDK5/p35:80 nM (IC50) Cdk1/cyclin B:320 nM (IC50) cdk2/cyclin A:300 nM (IC50) Cdk4/cyclin D1:10 μM (IC50) MAPKK:10 μM (IC50) protein kinase Cα:12 μM (IC50) |
| In Vitro | GSK 3 Inhibitor IX (BIO) is a specific inhibitor of glycogen synthase kinase-3 (GSK-3), with IC50 of 5 nM for GSK-3α/β, shows > 16-fold selectivity over CDK5. GSK 3 Inhibitor IX interacts within the ATP binding pocket of these kinases, reduces β-catenin phosphorylation on a GSK-3-specific site in cellular models, closely mimicks Wnt signaling in Xenopus embryos[1]. In human and mouse embryonic stem cells, GSK 3 Inhibitor IX (BIO) maintains the undifferentiated phenotype and sustains expression of the pluripotent state-specific transcription factors Oct-3/4, Rex-1 and Nanog. GSK 3 Inhibitor IX (BIO)-mediated Wnt activation is functionally reversible, as withdrawal of the compound leads to normal multidifferentiation programs in both human and mouse embryonic stem cells[2]. GSK 3 Inhibitor IX (BIO) promotes proliferation in mammalian cardiomyocytes[3]. GSK 3 Inhibitor IX (BIO) is also a pan-JAK inhibitor, with IC50 values of 0.03, 1.5, 8.0, 0.5 μM for TYK2, JAK1, JAK2 and JAK3, respectively. GSK 3 Inhibitor IX (BIO) selectively inhibits phosphorylation of STAT3 and induces apoptosis of human melanoma cells[4]. |
| In Vivo | GSK 3 Inhibitor IX (BIO) (50 mg/kg, p.o.) suppresses melanoma tumor growth in a mouse xenograft model[4]. |
| Cell Assay | COS1, Hepa (wild-type, CEM/LM AhR deficient and ELB1 ARNT deficient), or SH-SY5Y cells are grown in 6 cm culture dishes in Dulbecco's Modified Medium (DMEM) containing 10% fetal bovine serum. For treatment, IO (5 μM), GSK 3 Inhibitor IX (BIO) (5 or 10 μM), MeBIO (5 or 50 μM), LiCl (20 or 40 mM), or mock solution (DMSO, 0.5% final concentration) is added to medium when cell density reaches appr 70% confluence. After 12 (SH-SY5Y) or 24 hours, the cells, while still in plate, are lysed with lysis buffer (1% SDS, 1 mM sodium orthovanadate, 10 mM Tris [pH 7.4]). The lysate is passed several times through a 26G needle, centrifuged at 10,000× g for 5 min, and adjusted to equal protein concentration. About 8 μg of each sample is loaded for immunoblotting. Enhanced chemiluminescence is used for detection. The following primary antibodies are used: mouse anti-β-catenin CT, mouse anti-phospho-β-catenin, mouse anti-GSK-3 β, mouse anti-GSK-3 phosphoTyr216, rabbit anti-AhR (Aryl hydrocarbon receptor), and rabbit anti-actin. |
| Animal Admin | BALB/c mice (at 6-8 weeks old) and immunodeficient NOD/SCID/IL2Rgamma null (NSG) mice (female at 6-8 weeks old) are used in the assay. A2058 human melanoma cells at 5×106 cells in serum free medium are inoculated subcutaneously into the dorsal area of NSG mice to create xenograft model. When tumors become palpable, 6 GSK 3 Inhibitor IX (BIO) or vehicle control is administered via oral gavage once daily at 50 mg/kg body weight. Tumor growth is monitored every other day. Tumor volumes are measured every 3 to 4 days. Tumor volumes are calculated using the formula: 0.5 × (larger diameter) × (small diameter)2. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 554.3±50.0 °C at 760 mmHg |
| Melting Point | 300°C(lit.) |
| Molecular Formula | C16H10BrN3O2 |
| Molecular Weight | 356.173 |
| Flash Point | 289.0±30.1 °C |
| Exact Mass | 354.995636 |
| PSA | 73.72000 |
| LogP | 2.41 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.802 |
|
~75% 
BIO CAS#:667463-62-9 |
| Literature: Zhang, Aiying; Yu, Mingfeng; Lan, Tian; Liu, Zenglu; Mao, Zhenmin Synthetic Communications, 2010 , vol. 40, # 21 p. 3125 - 3134 |
|
~% 
BIO CAS#:667463-62-9 |
| Literature: Polychronopoulos, Panagiotis; Magiatis, Prokopios; Skaltsounis, Alexios-Leandros; Myrianthopoulos, Vassilios; Mikros, Emmanuel; Tarricone, Aldo; Musacchio, Andrea; Roe, S. Mark; Pearl, Laurence; Leost, Maryse; Greengard, Paul; Meijer, Laurent Journal of Medicinal Chemistry, 2004 , vol. 47, # 4 p. 935 - 946 |
|
~% 
BIO CAS#:667463-62-9 |
| Literature: Polychronopoulos, Panagiotis; Magiatis, Prokopios; Skaltsounis, Alexios-Leandros; Myrianthopoulos, Vassilios; Mikros, Emmanuel; Tarricone, Aldo; Musacchio, Andrea; Roe, S. Mark; Pearl, Laurence; Leost, Maryse; Greengard, Paul; Meijer, Laurent Journal of Medicinal Chemistry, 2004 , vol. 47, # 4 p. 935 - 946 |
|
~% 
BIO CAS#:667463-62-9 |
| Literature: Polychronopoulos, Panagiotis; Magiatis, Prokopios; Skaltsounis, Alexios-Leandros; Myrianthopoulos, Vassilios; Mikros, Emmanuel; Tarricone, Aldo; Musacchio, Andrea; Roe, S. Mark; Pearl, Laurence; Leost, Maryse; Greengard, Paul; Meijer, Laurent Journal of Medicinal Chemistry, 2004 , vol. 47, # 4 p. 935 - 946 |
|
Characterization of Leber Congenital Amaurosis-associated NMNAT1 Mutants.
J. Biol. Chem. 290 , 17228-38, (2015) Leber congenital amaurosis 9 (LCA9) is an autosomal recessive retinal degeneration condition caused by mutations in the NAD(+) biosynthetic enzyme NMNAT1. This condition leads to early blindness but n... |
|
|
Inhibitors of Matriptase-2 Based on the Trypsin Inhibitor SFTI-1.
ChemBioChem. 16 , 1601-7, (2015) A series of 17 new analogues of trypsin inhibitor SFTI-1 were designed and synthesized to obtain matriptase-2 inhibitors. A number of the modified bicyclic peptides displayed much higher affinity towa... |
|
|
Novel recA-Independent Horizontal Gene Transfer in Escherichia coli K-12.
PLoS ONE 10 , e0130813, (2015) In bacteria, mechanisms that incorporate DNA into a genome without strand-transfer proteins such as RecA play a major role in generating novelty by horizontal gene transfer. We describe a new illegiti... |
| 6-Bromoindirubin-3'-oxime |
| 6-bromoindirubin-3'-monoxime |
| (3Z)-6-Bromo-3-[(3E)-3-(hydroxyimino)-1,3-dihydro-2H-indol-2-ylidene]-1,3-dihydro-2H-indol-2-one |
| 2H-Indol-2-one, 6-bromo-3-[(3E)-1,3-dihydro-3-(hydroxyimino)-2H-indol-2-ylidene]-1,3-dihydro-, (3Z)- |
| (2'Z,3'E)-6-Bromoindirubin-3'-oxime |