Bethanechol
Modify Date: 2024-01-03 10:35:38
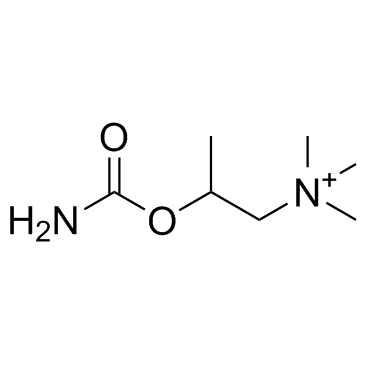
Bethanechol structure
|
Common Name | Bethanechol | ||
|---|---|---|---|---|
| CAS Number | 674-38-4 | Molecular Weight | 161.22200 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C7H17N2O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of BethanecholBethanechol is a parasympathomimetic choline carbamate that selectively stimulates muscarinic receptors without any effect on nicotinic receptors.Target: muscarinic receptorHyoscine butylbromide concentration dependently reduced muscle contractions, calcium mobilization, and epithelial secretion induced by the muscarinic agonist bethanechol with IC50 values of 429, 121, and 224 nmol L(-1), respectively.[2] Bethanechol presentes significantly improve of salivary parameters. Bethanechol is effective in decreasing the salivary gland damage.[3] Cerebral malakoplakia is a very rare chronic inflammatory disease. Treatment with antibiotic Bethanechol improves symptoms in association with a decrease in the abnormal calcification and enhancement.[4] |
| Name | bethanechol |
|---|---|
| Synonym | More Synonyms |
| Description | Bethanechol is a parasympathomimetic choline carbamate that selectively stimulates muscarinic receptors without any effect on nicotinic receptors.Target: muscarinic receptorHyoscine butylbromide concentration dependently reduced muscle contractions, calcium mobilization, and epithelial secretion induced by the muscarinic agonist bethanechol with IC50 values of 429, 121, and 224 nmol L(-1), respectively.[2] Bethanechol presentes significantly improve of salivary parameters. Bethanechol is effective in decreasing the salivary gland damage.[3] Cerebral malakoplakia is a very rare chronic inflammatory disease. Treatment with antibiotic Bethanechol improves symptoms in association with a decrease in the abnormal calcification and enhancement.[4] |
|---|---|
| Related Catalog | |
| References |
[1]. Bethanechol |
| Molecular Formula | C7H17N2O2 |
|---|---|
| Molecular Weight | 161.22200 |
| Exact Mass | 161.12900 |
| PSA | 52.32000 |
| LogP | 0.87670 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2924199090 |
|---|
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
| bethanechol |