Sulfadiazine
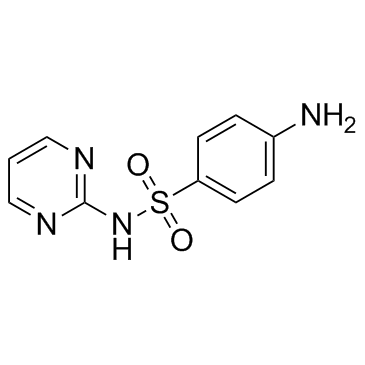
Sulfadiazine structure
|
Common Name | Sulfadiazine | ||
|---|---|---|---|---|
| CAS Number | 68-35-9 | Molecular Weight | 250.277 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 512.6±52.0 °C at 760 mmHg | |
| Molecular Formula | C10H10N4O2S | Melting Point | 253 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 263.8±30.7 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of SulfadiazineSulfadiazine is a sulfonamide antibiotic.Target: AntibacterialSulfadiazine eliminates bacteria that cause infections by stopping the production of folate inside the bacterial cell, and is commonly used to treat urinary tract infections (UTIs). In combination, sulfadiazine and pyrimethamine, can be used to treat toxoplasmosis, a disease caused by Toxoplasma gondii. From Wikipedia.The ulcers who treated with silver sulfadiazine cream responded rapidly, with one-third showing bacterial levels of less than 10(5) within three days, and half within a week [1]. |
| Name | sulfadiazine |
|---|---|
| Synonym | More Synonyms |
| Description | Sulfadiazine is a sulfonamide antibiotic.Target: AntibacterialSulfadiazine eliminates bacteria that cause infections by stopping the production of folate inside the bacterial cell, and is commonly used to treat urinary tract infections (UTIs). In combination, sulfadiazine and pyrimethamine, can be used to treat toxoplasmosis, a disease caused by Toxoplasma gondii. From Wikipedia.The ulcers who treated with silver sulfadiazine cream responded rapidly, with one-third showing bacterial levels of less than 10(5) within three days, and half within a week [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 512.6±52.0 °C at 760 mmHg |
| Melting Point | 253 °C (dec.)(lit.) |
| Molecular Formula | C10H10N4O2S |
| Molecular Weight | 250.277 |
| Flash Point | 263.8±30.7 °C |
| Exact Mass | 250.052444 |
| PSA | 106.35000 |
| LogP | -0.12 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.679 |
| Storage condition | 0-6°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H317-H319-H334-H335 |
| Precautionary Statements | P261-P280-P284-P304 + P340-P305 + P351 + P338-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn,Xi |
| Risk Phrases | R22 |
| Safety Phrases | S26-S36 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | WP1925000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2935009090 |
|
~99% 
Sulfadiazine CAS#:68-35-9 |
| Literature: Huang, Zhaohua; Wan, Decheng; Huang, Junlian Chemistry Letters, 2001 , # 7 p. 708 - 709 |
|
~% 
Sulfadiazine CAS#:68-35-9 |
| Literature: US2410793 , ; CH231191 , ; |
|
~% 
Sulfadiazine CAS#:68-35-9 |
| Literature: CH237327 , ; |
|
~% 
Sulfadiazine CAS#:68-35-9 |
| Literature: US2410793 , ; Dansk Tidsskrift for Farmaci, , vol. 16, p. 141,150 CH230861 , ; Journal of the American Chemical Society, , vol. 62, p. 2002,2003 Journal of the American Chemical Society, , vol. 64, p. 567,568 |
|
~% 
Sulfadiazine CAS#:68-35-9 |
| Literature: Journal of Medicinal Chemistry, , vol. 57, # 2 p. 495 - 506 |
|
~% 
Sulfadiazine CAS#:68-35-9 |
| Literature: Yakugaku Zasshi, , vol. 70, p. 283,284 Chem.Abstr., , p. 2894 |
|
~% 
Sulfadiazine CAS#:68-35-9 |
| Literature: Yakugaku Zasshi, , vol. 71, p. 1420 Chem.Abstr., , p. 8095 |
|
~% 
Sulfadiazine CAS#:68-35-9 |
| Literature: DE743007 , ; DRP/DRBP Org.Chem. |
| Precursor 9 | |
|---|---|
| DownStream 5 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
|
|
Convenient QSAR model for predicting the complexation of structurally diverse compounds with β-cyclodextrins
Bioorg. Med. Chem. 17 , 896-904, (2009) This paper reports a QSAR study for predicting the complexation of a large and heterogeneous variety of substances (233 organic compounds) with beta-cyclodextrins (beta-CDs). Several different theoret... |
| Sulfadiazine |
| A-306 |
| 2-sulfanilylaminopyrimidine |
| 2-sulfanilamidopyrimidine |
| PYRIMAL |
| FLAMAZINE |
| sulfapyrimidine |
| MFCD00006065 |
| Silvadene |
| sulphadiazine |
| Adiazine |
| EINECS 200-685-8 |
| 4-amino-N-(pyrimidin-2-yl)benzenesulfonamide |
| DEBENAL |
| 4-Amino-N-pyrimidin-2-yl-benzenesulfonamide |
| Ultradiazin |
| Benzenesulfonamide, 4-amino-N-2-pyrimidinyl- |
| Adiazin |
| rp2616 |
| N1-2-Pyrimidinylsulfanilamide |
| 4-Amino-N-(2-pyrimidinyl)benzenesulfonamide |
| Diazin |
| DIAZYL |
| N1-2-Pyrimidylsulfanilamide |
| SD-Na |
| Trisem |


![Benzenesulfonamide,4-[2-(1-acetyl-2-oxopropyl)diazenyl]-N-2-pyrimidinyl- structure](https://image.chemsrc.com/caspic/069/977-20-8.png) CAS#:977-20-8
CAS#:977-20-8 CAS#:64047-46-7
CAS#:64047-46-7![4-[(6-oxo-1-cyclohexa-2,4-dienylidene)methylamino]-N-pyrimidin-2-yl-benzenesulfonamide structure](https://image.chemsrc.com/caspic/412/5440-97-1.png) CAS#:5440-97-1
CAS#:5440-97-1![3-[[4-(pyrimidin-2-ylsulfamoyl)phenyl]carbamoyl]propanoic acid structure](https://image.chemsrc.com/caspic/400/40266-03-3.png) CAS#:40266-03-3
CAS#:40266-03-3
