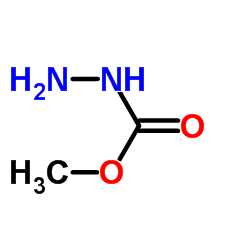
Carbadox
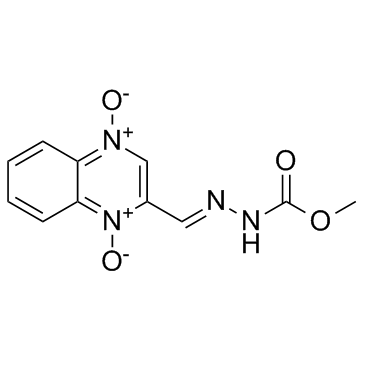
Carbadox structure
|
Common Name | Carbadox | ||
|---|---|---|---|---|
| CAS Number | 6804-07-5 | Molecular Weight | 262.22200 | |
| Density | 1.447g/cm3 | Boiling Point | 405.47°C (rough estimate) | |
| Molecular Formula | C11H10N4O4 | Melting Point | 239-240ºC | |
| MSDS | Chinese USA | Flash Point | 18°(64°F) | |
| Symbol |



GHS02, GHS07, GHS08 |
Signal Word | Danger | |
Use of CarbadoxCarbadox is a quinoxaline-di-N-oxide antibiotic compound which is widely fed to nursery-age pigs to control enteric diseases and improve feed efficiency. |
| Name | Carbadox |
|---|---|
| Synonym | More Synonyms |
| Description | Carbadox is a quinoxaline-di-N-oxide antibiotic compound which is widely fed to nursery-age pigs to control enteric diseases and improve feed efficiency. |
|---|---|
| Related Catalog | |
| Target |
Bacterial[1] |
| In Vitro | The results of MTT assay demonstrate a dose-dependent decrease in mitochondrial activity in Vero cells at all concentrations of Carbadox. Treatment with Carbadox at the highest concentration of 160 μg/mL results in cell viability down to only 12%. Cells following Carbadox treatment show a dose-dependent increase of the DNA migration (p<0.01). The nuclear division index (NDI) reduces markedly with the increase doses of Carbadox[1]. |
| In Vivo | Alpha diversities (Shannon diversity, Heips evenness, and inverse Simpson indices) of samples from medicated piglets compare to non-medicated piglets are significantly different at 2, 3, and 4 days after continuous Carbadox, but not different in either late Carbadox or at any time during the withdrawal period. Analysis of the community structure of bacteria in animals shows significant differences at days 3 and 4 of early Carbadox treatment ([R=0.32, p=0.015] and [R=0.54, p=0.003], respectively), but not before starting antibiotic treatment (p=0.82). No significant differences in E. coli colony forming units (CFUs) are observed during the Carbadox-treatment period of the study or late in the withdrawal period. E. coli CFUs are significantly different between the medicated and non-medicated groups on day 2 after the withdrawal of Carbadox[2]. |
| Cell Assay | Exponentially growing Vero cells are seeded at 104 cells/well density in 96 microplates and exposed to various concentrations of Carbadox (5, 10, 20, 40, 80, 160, 210, 260, 310 and 360 μg/mL). Cells incubated with the same concentration DMSO are used as a control. After 4 h or 24 h, each well is added 100 μL MTT solution (200 μg/mL) followed incubation for 4 h at 37 °C, and the medium containing MTT is removed. The formazan crystals in the viable cells are solubilized with 100 μL DMSO and the absorbance at 570 nm of each well is read using a microplate reader. All experiments are performed at least 3 times, with 6 wells for each concentration of Carbadox (n=6 per experiment). Final results are the average of three independent experiments. The cell viability is calculated as follows: OD of experimental group/(OD of control group-OD of blank group)×100%. The data are presented as means±SE[1]. |
| Animal Admin | At 3 weeks of age, 12 piglets from 2 litters are divided into two rooms of six pigs each, with equal representation of littermates and gender. All pigs are fed a standard starter diet ad libitum for 3 weeks, after which six control pigs continue to receive non-medicated feed while the other group receives feed containing Carbadox (50 g/ton). After 21 days of continuous feed with or without Carbadox, all pigs (60 days old) are switched to a non-medicated maintenance diet. Feces are collected from each pig at multiple times before, during, and after antibiotic withdrawal[2]. |
| References |
| Density | 1.447g/cm3 |
|---|---|
| Boiling Point | 405.47°C (rough estimate) |
| Melting Point | 239-240ºC |
| Molecular Formula | C11H10N4O4 |
| Molecular Weight | 262.22200 |
| Flash Point | 18°(64°F) |
| Exact Mass | 262.07000 |
| PSA | 101.61000 |
| LogP | 1.77760 |
| Index of Refraction | 1.648 |
| Storage condition | 2-8°C |
| Water Solubility | Soluble in 1N NaOH (50 mg/ml), and water (partly). |
| Symbol |



GHS02, GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H228-H302-H350 |
| Precautionary Statements | P201-P210-P308 + P313 |
| Hazard Codes | F:Flammable |
| Risk Phrases | R45;R11;R22 |
| Safety Phrases | S53-S45 |
| RIDADR | UN 1325 4.1/PG 2 |
| WGK Germany | 3 |
| RTECS | FE2779000 |
| HS Code | 29339900 |
|
~95% 
Carbadox CAS#:6804-07-5 |
| Literature: Haddadin, Makhluf J.; Kattan, Asma M. A.; Issidorides, Costas H. Journal of Organic Chemistry, 1985 , vol. 50, # 1 p. 129 - 130 |
|
~% 
Carbadox CAS#:6804-07-5 |
| Literature: Journal of Organic Chemistry, , vol. 50, # 1 p. 129 - 130 |
|
~% 
Carbadox CAS#:6804-07-5 |
| Literature: Journal of Organic Chemistry, , vol. 50, # 1 p. 129 - 130 |
|
Reduction of carbadox mediated by reaction of Mn(III) with oxalic acid.
Environ. Sci. Technol. 47(3) , 1357-64, (2013) Manganese(III) geocomponents are commonly found in the soil environment, yet their roles in many biogeochemical processes remain unknown. In this study, we demonstrated that Mn(III) generated from the... |
|
|
Determination of amprolium, carbadox, monensin, and tylosin in surface water by liquid chromatography/tandem mass spectrometry.
Rapid Commun. Mass Spectrom. 21(12) , 1944-50, (2007) Antibiotics present in the environment are recently considered as emerging contaminants, and have raised increasing concerns about their potential risks to ecosystems and human health. In addition to ... |
|
|
Antibiotics in feed induce prophages in swine fecal microbiomes.
MBio 2(6) , doi:10.1128/mBio.00260-11, (2011) Antibiotics are a cost-effective tool for improving feed efficiency and preventing disease in agricultural animals, but the full scope of their collateral effects is not understood. Antibiotics have b... |
| fortigro |
| (1,4-dioxy-quinoxalin-2-ylmethylene)-hydrazinecarboxylic acid methyl ester |
| methyl-3-(2-quinoxalinylmethylene)carbazate N,N'-dioxide |
| karbadox |
| carabadox |
| methyl 3-(2-quinoxalinylmethylene)carbazate N1,N4-dioxide |
| Mecadox-S |
| Mecadox |
| CARBADO |
| getroxel |
| gs6244 |
| EINECS 229-879-0 |
| MFCD00057293 |