4-hydroxytamoxifen
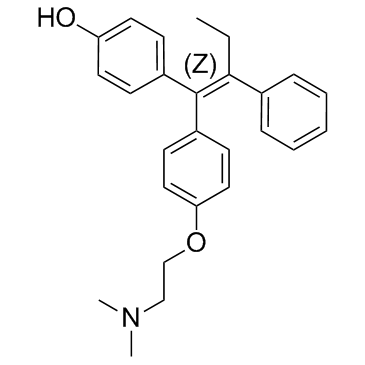
4-hydroxytamoxifen structure
|
Common Name | 4-hydroxytamoxifen | ||
|---|---|---|---|---|
| CAS Number | 68047-06-3 | Molecular Weight | 387.514 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 514.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C26H29NO2 | Melting Point | 105-107ºC | |
| MSDS | Chinese USA | Flash Point | 264.9±30.1 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of 4-hydroxytamoxifen4-Hydroxytamoxifen is a selective estrogen receptor modulator (SERM). |
| Name | afimoxifene |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Hydroxytamoxifen is a selective estrogen receptor modulator (SERM). |
|---|---|
| Related Catalog | |
| Target |
Estrogen receptor:3.3 nM (IC50) CRISPR/Cas9 |
| In Vitro | 4-Hydroxytamoxifen (Monohydroxytamoxifen) is a selective oestrogen receptor antagonist, with an IC50 of 3.3 nM for the [3H]oestradiol binding to oestrogen receptor. 4-Hydroxytamoxifen (10, 100 nM) enables to inhibit the binding of [3H]oestradiol to the human 8 S oestrogen receptor[1]. 4-Hydroxytamoxifen activates intein-linked inactive Cas9, reduces off-target CRISPR-mediated gene editing. In human cells, conditionally active Cas9s modify target genomic sites with up to 25-fold higher specificity than wild-type Cas9[2]. |
| In Vivo | 4-Hydroxytamoxifen (0.2, 1 and 5 μg/day, p.o.) causes a dose-related decrease in uterine wet weight of immature rats[1]. 4-Hydroxytamoxifen (6 μg/0.1 mL sesame oil/day, s.c.) effectively attenuates methamphetamine-induced nigrostriatal dopamine depletions in bothsexes of intact and gonadectomized C57BL/6 J mice. 4-Hydroxytamoxifen does not alter the dopamine content levels in the striatum[3]. |
| Kinase Assay | Cytosol (200 μL) is incubated for 30 min at 4°C with different concentrations of oestradiol, tamoxifen and (4-Hydroxytamoxifen) or dihydroxytamoxifen administered in 10 μL methanol. Control tubes are incubated with 10 μL methanol alone and non-specific binding is determined in a parallel incubation of cytosol (200 μL) with methanol (10 μL) containing DES (5 × 106 M). [2,4,6,7-3H]Oestradiol solution (50 μL) in TED buffer is added to each tube to give a final concentration of 2 × 10-9 M. Incubation is continued for 4 h (4°C) and then 400 μL of a suspension of dextran-coated charcoal (250 mg % Norit A, 2.5 mg % dextran) in TED buffer are added and allowed to stand for 20 min. Tubes are centrifuged at 800 g for 10 min (4°C) and 400 μL samples of the supernatant are added to 10 mL tritium scintillator (6 g butyl PBD, 135 mL toluene, 720 ml dioxan, 100 g naphthalene, 45 mL absolute methanol). Samples are counted for 10 min in a liquid scintillation spectrometer. Counting efficiency is determined by external standardization (35-36 %). Results are represented as a percentage of the specifically bound radioactivity (c.p.m.) in the control tubes[1]. |
| Animal Admin | Mice[3] Animals of each sex are divided into two groups: one group receives 4-Hydroxytamoxifen [6 μg/0.1 mL sesame oil/day, subcutaneously (s.c.) starting at 06.00 h] injections for three consecutive days, while the other group receives an equivalent amount of sesame oil injection for 3 days. Four hours following the third injection, each group is then subdivided into two groups: one receives four cumulative doses of methamphetamine hydrochloride (10 mg/kg, s.c.), and the other receives a comparable volume of saline at 2-h intervals. Bilateral gonadectomy is performed under pentobarbital anesthesia (50 mg/kg, intraperitoneally). Five weeks after surgery,gonadectomized mice of each sex are randomly divided into six groups. Five groups of each sex receive three daily injections ofvarious concentrations of 4-Hydroxytamoxifen (0, 1.5, 3.0, 6.0, and 12.0 μg/0.1 mL sesame oil/day). Four hours following the third injection, mice receive four doses of methamphetamine (MA, 10 mg/kg) at 2-h intervals. The remaining group of each sex receives sesame oil pretreatment for three consecutive days, followed by saline injections, and serves as the control group[3]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 514.4±50.0 °C at 760 mmHg |
| Melting Point | 105-107ºC |
| Molecular Formula | C26H29NO2 |
| Molecular Weight | 387.514 |
| Flash Point | 264.9±30.1 °C |
| Exact Mass | 387.219818 |
| PSA | 32.70000 |
| LogP | 7.34 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.597 |
| Storage condition | 2-8°C |
| Stability | Stable. Store cool. Incompatible with strong oxidizing agents. |
| Water Solubility | 95% ethanol: 20 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H332-H361 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn |
| Risk Phrases | R20/21/22;R63 |
| Safety Phrases | S22-S23-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | SL1210000 |
| Precursor 8 | |
|---|---|
| DownStream 2 | |
|
Inhibitory mechanism of FAT4 gene expression in response to actin dynamics during Src-induced carcinogenesis.
PLoS ONE 10(2) , e0118336, (2015) Oncogenic transformation is characterized by morphological changes resulting from alterations in actin dynamics and adhesive activities. Emerging evidence suggests that the protocadherin FAT4 acts as ... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Impact of induced fit on ligand binding to the androgen receptor: a multidimensional QSAR study to predict endocrine-disrupting effects of environmental chemicals.
J. Med. Chem. 48 , 5666-74, (2005) We investigated the influence of induced fit of the androgen receptor binding pocket on free energies of ligand binding. On the basis of a novel alignment procedure using flexible docking, molecular d... |
| MFCD00278780 |
| 4-hydroxytamoxifen |
| 4-[(1Z)-1-{4-[2-(Dimethylamino)ethoxy]phenyl}-1-phenyl-1-buten-2-yl]phenol |
| Phenol, 4-[(1Z)-1-[[4-[2-(dimethylamino)ethoxy]phenyl]phenylmethylene]propyl]- |
| 4-{(1Z)-1-[{4-[2-(dimethylamino)ethoxy]phenyl}(phenyl)methylidene]propyl}phenol |
| 4-{(1Z)-1-[{4-[2-(dimethylamino)ethoxy]phenyl}(phenyl)methylene]propyl}phenol |
| 4-hydroxy-tamoxifen |
![(E)-1-[4-(benzyloxy)phenyl]-1-[4-(2-dimethylaminoethoxy)phenyl]-2-phenylbut-1-ene Structure](https://image.chemsrc.com/caspic/436/109517-76-2.png) CAS#:109517-76-2
CAS#:109517-76-2 CAS#:124-40-3
CAS#:124-40-3 CAS#:611-99-4
CAS#:611-99-4 CAS#:68047-08-5
CAS#:68047-08-5 CAS#:93-55-0
CAS#:93-55-0![(E,Z)-1-[4-(benzyloxy)phenyl]-1-(4-hydroxyphenyl)-2-phenylbut-1-ene Structure](https://image.chemsrc.com/caspic/447/185223-78-3.png) CAS#:185223-78-3
CAS#:185223-78-3![(E)-1-[4-(benzyloxy)phenyl]-1-(4-hydroxyphenyl)-2-phenylbut-1-ene Structure](https://image.chemsrc.com/caspic/396/440646-09-3.png) CAS#:440646-09-3
CAS#:440646-09-3![4-benzyloxy-4'-[(p-perfluorotolyl)oxy]benzophenone Structure](https://image.chemsrc.com/caspic/196/1028310-31-7.png) CAS#:1028310-31-7
CAS#:1028310-31-7![[4-[(E)-1-[4-[2-(dimethylamino)ethoxy]phenyl]-2-phenylbut-1-enyl]phenyl] acetate structure](https://image.chemsrc.com/caspic/362/76117-70-9.png) CAS#:76117-70-9
CAS#:76117-70-9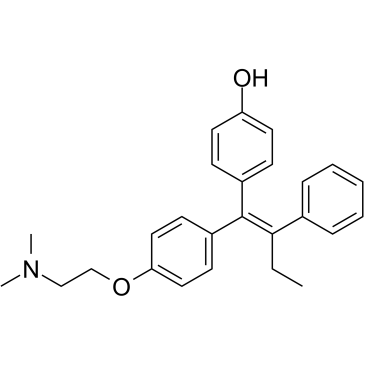 CAS#:174592-47-3
CAS#:174592-47-3