2-Bromoacetamide

2-Bromoacetamide structure
|
Common Name | 2-Bromoacetamide | ||
|---|---|---|---|---|
| CAS Number | 683-57-8 | Molecular Weight | 137.963 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 271.6±23.0 °C at 760 mmHg | |
| Molecular Formula | C2H4BrNO | Melting Point | 87-91 °C(lit.) | |
| MSDS | USA | Flash Point | 118.1±22.6 °C | |
| Symbol |


GHS05, GHS06 |
Signal Word | Danger | |
Use of 2-Bromoacetamide2-Bromoacetamide can inactivate alcohol dehydrogenase[1]. |
| Name | 2-Bromoacetamide |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Bromoacetamide can inactivate alcohol dehydrogenase[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 271.6±23.0 °C at 760 mmHg |
| Melting Point | 87-91 °C(lit.) |
| Molecular Formula | C2H4BrNO |
| Molecular Weight | 137.963 |
| Flash Point | 118.1±22.6 °C |
| Exact Mass | 136.947617 |
| PSA | 43.09000 |
| LogP | -0.52 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.511 |
| Storage condition | Store at RT. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS05, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H314 |
| Precautionary Statements | P280-P301 + P310-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T,C |
| Risk Phrases | R25;R34 |
| Safety Phrases | S26-S27-S36/37/39-S45 |
| RIDADR | UN 2923 8/PG 2 |
| WGK Germany | 3 |
| RTECS | AB4587000 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2924199013 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924199013 |
|---|---|
| Summary | HS:2924199013 2-bromoacetamide VAT:17.0% Tax rebate rate:0.0% Supervision conditions:S MFN tariff:6.5% General tariff:30.0% |
|
Carborane-derived local anesthetics are isomer dependent.
ChemMedChem 10(1) , 62-7, (2014) Clinically there is a need for local anesthetics with a greater specificity of action on target cells and longer duration. We have synthesized a series of local anesthetic derivatives we call boronica... |
|
|
(N)-methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists.
J. Med. Chem. 48(6) , 1745-58, (2005) A series of ring-constrained (N)-methanocarba-5'-uronamide 2,N(6)-disubstituted adenine nucleosides have been synthesized via Mitsunobu condensation of the nucleobase precursor with a pseudosugar ring... |
|
|
Kinetic analysis of sequence-specific alkylation of DNA by pyrimidine oligodeoxyribonucleotide-directed triple-helix formation.
Bioconjug. Chem. 8(3) , 354-64, (1997) Attachment of a nondiffusible bromoacetyl electrophile to the 5-position of a thymine at the 5'-end of a pyrimidine oligodeoxyribonucleotide affords sequence-specific alkylation of a guanine base in d... |
| Bromoacetamide |
| EINECS 226-270-1 |
| Acetamide, 2-bromo- |
| MFCD00008025 |
| 2-Bromoacetamide |
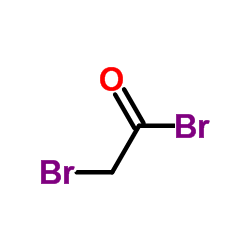 CAS#:598-21-0
CAS#:598-21-0 CAS#:22118-09-8
CAS#:22118-09-8 CAS#:60311-02-6
CAS#:60311-02-6 CAS#:463-51-4
CAS#:463-51-4 CAS#:35070-76-9
CAS#:35070-76-9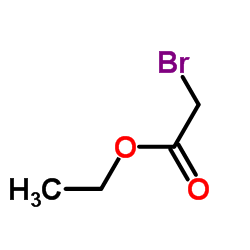 CAS#:105-36-2
CAS#:105-36-2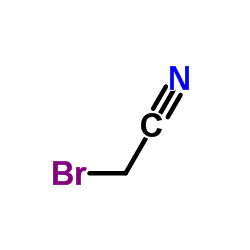 CAS#:590-17-0
CAS#:590-17-0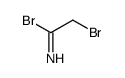 CAS#:710272-17-6
CAS#:710272-17-6 CAS#:110-83-8
CAS#:110-83-8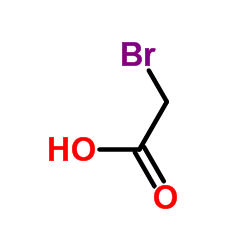 CAS#:79-08-3
CAS#:79-08-3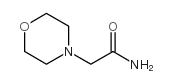 CAS#:5625-98-9
CAS#:5625-98-9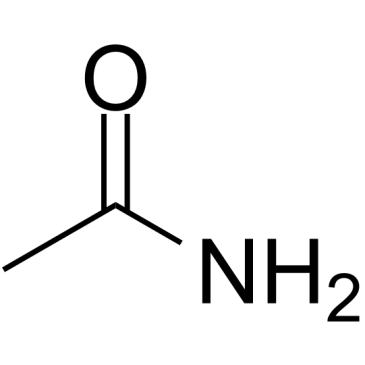 CAS#:60-35-5
CAS#:60-35-5![2-[(Diphenylmethyl)Thio]Acetamide structure](https://image.chemsrc.com/caspic/041/68524-30-1.png) CAS#:68524-30-1
CAS#:68524-30-1 CAS#:1005785-49-8
CAS#:1005785-49-8 CAS#:1003011-27-5
CAS#:1003011-27-5 CAS#:157599-02-5
CAS#:157599-02-5 CAS#:4774-22-5
CAS#:4774-22-5 CAS#:345612-63-7
CAS#:345612-63-7 CAS#:35382-75-3
CAS#:35382-75-3
