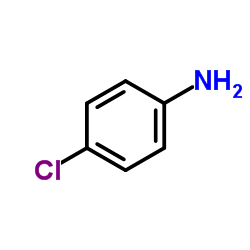
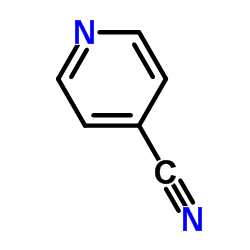
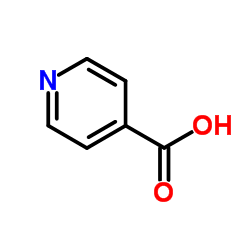
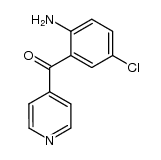
Vercirnon
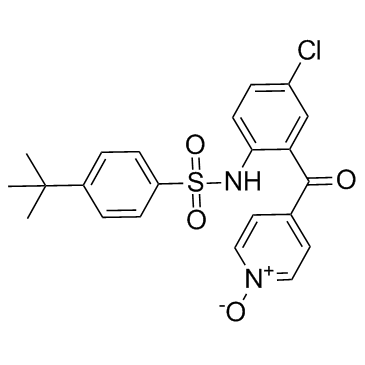
Vercirnon structure
|
Common Name | Vercirnon | ||
|---|---|---|---|---|
| CAS Number | 698394-73-9 | Molecular Weight | 444.93100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C22H21ClN2O4S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of VercirnonVercirnon is an orally bioavailable, selective, and potent antagonist of CCR9, with an IC50 of 10 nM, used in the research of inflammatory bowel diseases. |
| Name | 4-tert-butyl-N-[4-chloro-2-(1-oxidopyridin-1-ium-4-carbonyl)phenyl]benzenesulfonamide |
|---|---|
| Synonym | More Synonyms |
| Description | Vercirnon is an orally bioavailable, selective, and potent antagonist of CCR9, with an IC50 of 10 nM, used in the research of inflammatory bowel diseases. |
|---|---|
| Related Catalog | |
| Target |
CCR9:10 nM (IC50) |
| In Vitro | Vercirnon (CCX282-B) is an orally bioavailable, selective, and potent antagonist of human CCR9. Vercirnon inhibits CCL25-induced calcium mobilization with an IC50 value of 5.4 ± 0.7 nM. Vercirnon is also a potent inhibitor of CCL25-induced Molt-4 chemotaxis with an IC50 of 3.5 ± 0.3 nM. Vercirnon shows inhibitory activity against Molt-4 migration with an IC50 of 33.4 ± 1.3 nM in 100% human AB serum. Moreover, Vercirnon suppresses the binding of [3H] CCX807 to Molt-4 cells with an IC50 of 6 nM. Vercirnon inhibits the chemotaxis of Baf-3/CCR9A cells as well as Baf-3/CCR9A cells with IC50s of 2.8 ± 1.1 nM and 2.6 ± 0.7 nM, respectively. Vercirnon potently inhibits CCL25-induced chemotaxis with an IC50 value of 6.8 ± 1.7 nM in buffer, and exhibits inhibition on RA-cultured cell CCL25-medidated chemotaxis in 100% human AB serum with an IC50 of 141 ± 13 nM[1]. |
| In Vivo | Vercirnon (10, 50 mg/kg twice daily) protects from the severe inflammation associated with TNF-α overexpression in the TNFΔARE Mouse Model. Vercirnon (50 mg/kg c.c. twice daily) blocks the colitis-associated weight loss inherent in the mdr1a−/− model, and also abrogates growth arrest in the colitis mdr1a −/− mice[2]. |
| Cell Assay | Cells are harvested by centrifugation and resuspended in chemotaxis buffer consisting of HBSS with 0.1% BSA at a density of 107 cells/mL. For assays designed to determine Vercirnon potency in the presence of human serum, cells are resuspended in 100% human AB serum from pooled donors. Equal volumes of cell suspension and diluted Vercirnon are mixed and incubated for 10 min at room temperature. Twenty microliters of the mixture is transferred to the upper chamber of the chemotaxis chamber. After a 120-min incubation at 37°C, the assay is terminated by removal of cell drops from the top of the filter. Migration signal is determined by adding 5 μL of CyQUANT solution to each well in the lower chemotaxis chamber and measuring the intensity of fluorescence on a Spectrafluor Plus plate reader[1]. |
| Animal Admin | Mice[2] Female mdr1a−/− and wild-type FVB mice are used in the assay. CCX025, formulated in 1% hydroxypropyl methylcellulose, is dosed at 100 mg/kg s.c. once daily. Vercirnon, formulated in 5% Cremophor, is dosed at 50 mg/kg c.c. twice daily. During the course of the study, body weights and the incidence of diarrhea are recorded on a weekly basis. Any animals that exhibits weight loss of greater than 20% of their peak body weight are euthanized. Final body weights for any euthanized or dead animals are carried forward for data analysis. Diarrhea is scored on a 0-5 scale; when animals reach a score of ≥3, their diarrhea is constant and irreversible and is thus considered as established diarrhea[2]. |
| References |
| Molecular Formula | C22H21ClN2O4S |
|---|---|
| Molecular Weight | 444.93100 |
| Exact Mass | 444.09100 |
| PSA | 97.08000 |
| LogP | 6.25160 |
| Storage condition | 2-8℃ |
|
~% 
Vercirnon CAS#:698394-73-9 |
| Literature: CHEMOCENTRYX Patent: WO2004/46092 A2, 2004 ; Location in patent: Page 63-64 ; WO 2004/046092 A2 |
|
~% 
Vercirnon CAS#:698394-73-9 |
| Literature: Takeda Pharmaceutical Company Limited; Suzuki, Nobutaka; Miyazaki, Takahiro; Ochi, Takashi Patent: US2014/155437 A1, 2014 ; |
|
~% 
Vercirnon CAS#:698394-73-9 |
| Literature: Takeda Pharmaceutical Company Limited; Suzuki, Nobutaka; Miyazaki, Takahiro; Ochi, Takashi Patent: US2014/155437 A1, 2014 ; |
|
~% 
Vercirnon CAS#:698394-73-9 |
| Literature: Takeda Pharmaceutical Company Limited; Suzuki, Nobutaka; Miyazaki, Takahiro; Ochi, Takashi Patent: US2014/155437 A1, 2014 ; |
|
~% 
Vercirnon CAS#:698394-73-9 |
| Literature: Takeda Pharmaceutical Company Limited; Suzuki, Nobutaka; Miyazaki, Takahiro; Ochi, Takashi Patent: US2014/155437 A1, 2014 ; |
|
~% 
Vercirnon CAS#:698394-73-9 |
| Literature: Takeda Pharmaceutical Company Limited; Suzuki, Nobutaka; Miyazaki, Takahiro; Ochi, Takashi Patent: US2014/155437 A1, 2014 ; |
|
~% 
Vercirnon CAS#:698394-73-9 |
| Literature: Takeda Pharmaceutical Company Limited; Suzuki, Nobutaka; Miyazaki, Takahiro; Ochi, Takashi Patent: US2014/155437 A1, 2014 ; |
|
~% 
Vercirnon CAS#:698394-73-9 |
| Literature: Takeda Pharmaceutical Company Limited; Suzuki, Nobutaka; Miyazaki, Takahiro; Ochi, Takashi Patent: US2014/155437 A1, 2014 ; |
| UNII-MWI54OUA12 |
| 4-tert-butyl-N-[4-chloro-2-(1-oxy-pyridine-4-carbonyl)-phenyl]-benzenesulfonamide |
| 4-tert-butyl-N-[4-chloro-2-(1-oxo-pyridine-4-carbonyl)-phenyl]-benzenesulfonamide |
| Vercirnon |
| N-{4-chloro-2-[(1-oxidopyridin-4-yl)carbonyl]phenyl}-4-(tert-butyl)benzenesulfonamide |
![4-tert-butyl-N-[4-chloro-2-(pyridine-4-carbonyl)-phenyl]-benzenesulfonamide structure](https://image.chemsrc.com/caspic/342/698394-72-8.png)