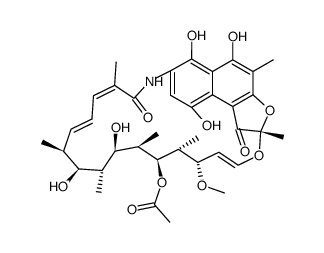
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KD1922000
-
CHEMICAL NAME :
-
2,7-(Epoxypentadeca(1,11,13)trienimino)naphtho(2,1-b) furan-1,11(2H)-dione, 5,6,9,17,19,21-hexahydroxy-23-methoxy-2,4,12,16,18,20 ,22-heptamethyl-, 21-acetate
-
CAS REGISTRY NUMBER :
-
6998-60-3
-
LAST UPDATED :
-
199509
-
DATA ITEMS CITED :
-
11
-
MOLECULAR FORMULA :
-
C37-H47-N-O12
-
MOLECULAR WEIGHT :
-
697.85
-
WISWESSER LINE NOTATION :
-
T C6 B65-24- A D E 2BC G& AV LO NO F&VM OU B&U D&U MH&&&TJ DQ GQ IQ J1 M1 QO1 R1 SOV1 T1 UQ V
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2120 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
625 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
450 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intracerebral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
5800 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
700 mg/kg
-
SEX/DURATION :
-
female 7-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
500 mg/kg
-
SEX/DURATION :
-
female 2 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
900 mg/kg
-
SEX/DURATION :
-
female 8-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1800 mg/kg
-
SEX/DURATION :
-
female 8-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
MUTATION DATA
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TEST SYSTEM :
-
Rodent - mouse Mammary gland
-
DOSE/DURATION :
-
6300 nmol/L/24H (Continuous)
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 5,141,1980 *** REVIEWS *** TOXICOLOGY REVIEW ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 15,951,1965
|