H-Gly-Pro-OH
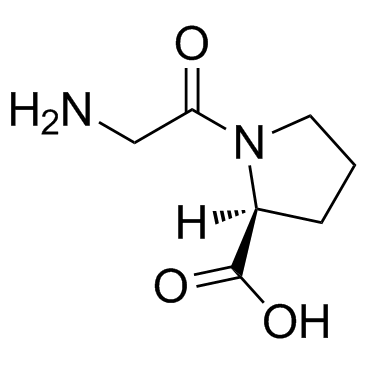
H-Gly-Pro-OH structure
|
Common Name | H-Gly-Pro-OH | ||
|---|---|---|---|---|
| CAS Number | 704-15-4 | Molecular Weight | 172.182 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 411.3±40.0 °C at 760 mmHg | |
| Molecular Formula | C7H12N2O3 | Melting Point | 185℃ | |
| MSDS | Chinese USA | Flash Point | 202.6±27.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of H-Gly-Pro-OHH-Gly-Pro-OH is an end product of collagen metabolism that is further cleaved by prolidase. |
| Name | Gly-Pro |
|---|---|
| Synonym | More Synonyms |
| Description | H-Gly-Pro-OH is an end product of collagen metabolism that is further cleaved by prolidase. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
[1]. Le J, et al. Urine glycyl-L-proline increase and skin trophicity. Amino Acids. 1999;17(3):315-22. |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 411.3±40.0 °C at 760 mmHg |
| Melting Point | 185℃ |
| Molecular Formula | C7H12N2O3 |
| Molecular Weight | 172.182 |
| Flash Point | 202.6±27.3 °C |
| Exact Mass | 172.084793 |
| PSA | 83.63000 |
| LogP | -1.34 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.557 |
| Storage condition | −20°C |
| Precursor 9 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease.
J. Chromatogr. A. 562(1-2) , 125-38, (1991) Eighty-five clinical urine samples and nineteen urine samples previously found by other laboratories to suggest genetic metabolic defects were prepared for trimethylsilylation by treatment with urease... |
|
|
Highly sensitive GC/MS/MS method for quantitation of amino and nonamino organic acids.
Anal. Chem. 83(7) , 2705-11, (2011) Metabolite profiling methods are important tools for measurement of metabolite pools in biological systems. While most metabolite profiling methods report relative intensities or depend on a few inter... |
|
|
Diagnostic use of cerebral and extracerebral oxysterols.
Clin. Chem. Lab Med. 42(2) , 186-91, (2004) 24S-Hydroxycholesterol (24OHC) and 27-hydroxycholesterol (27OHC) are two structurally similar oxysterols of different origins--the former almost exclusively formed in the brain and the latter formed t... |
| MFCD00020840 |
| Glycyl-L-proline |
| 1-glycyl-L-Proline |
| Proline, 1-glycyl-, L- |
| L-Proline, glycyl- |
| (2S)-1-(Aminoacetyl)-2-pyrrolidinecarboxylic acid |
| 1-Glycyl-L-prolin |
| Gly-l-pro |
| L-Proline, 1-glycyl- |
| EINECS 211-880-2 |
| H-Gly-Pro-OH |
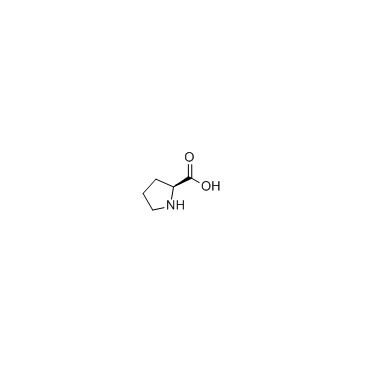 CAS#:147-85-3
CAS#:147-85-3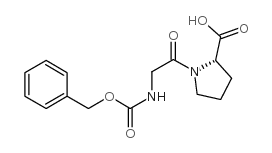 CAS#:1160-54-9
CAS#:1160-54-9 CAS#:212651-48-4
CAS#:212651-48-4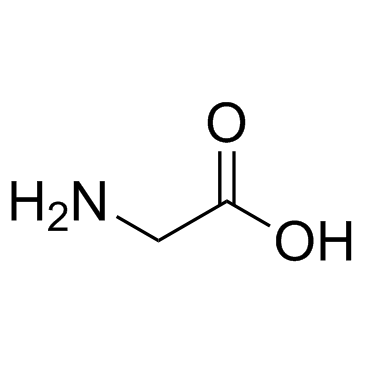 CAS#:56-40-6
CAS#:56-40-6 CAS#:23500-10-9
CAS#:23500-10-9 CAS#:60189-43-7
CAS#:60189-43-7 CAS#:2646-61-9
CAS#:2646-61-9 CAS#:3705-27-9
CAS#:3705-27-9 CAS#:33256-35-8
CAS#:33256-35-8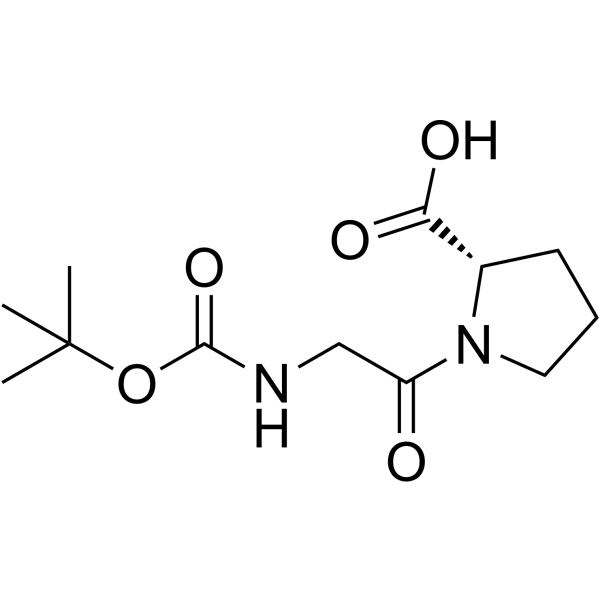 CAS#:14296-92-5
CAS#:14296-92-5
