Cloxacillin Sodium
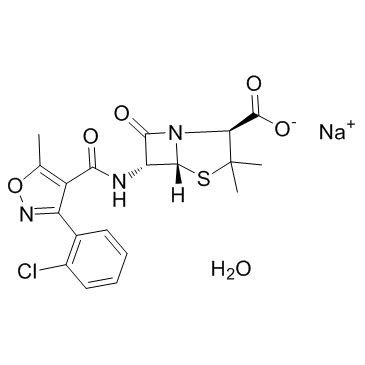
Cloxacillin Sodium structure
|
Common Name | Cloxacillin Sodium | ||
|---|---|---|---|---|
| CAS Number | 7081-44-9 | Molecular Weight | 475.88 | |
| Density | N/A | Boiling Point | 689.7ºC at 760 mmHg | |
| Molecular Formula | C19H19ClN3NaO6S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 370.9ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of Cloxacillin SodiumCloxacillin sodium monohydrate is a semi-synthetic antibiotic that is a chlorinated derivative of oxacillin.Target: AntibacterialCloxacillin sodium (Cloxacap) is a sodium salt of cloxacillin that is a penicillinase-resistant, acid resistant, semi-synthetic penicillin. Cloxacillin sodium exerts a bactericidal action against susceptible microorganisms during the stage of active multiplication. Cloxacillin sodium acts through the inhibition of biosynthesis of cell wall mucopeptides. Cloxacillin sodium is readily absorbed following i.m. administration and rapidly reaches therapeutically effective blood levels. Serum levels are approximately proportional to dosage. Peak plasma concentrations of 15 ug/ml have been observed 30 minutes after an i.m. injection of cloxacillin (Cloxapen, Cloxacap and Orbenin) 500 mg; plasma concentrations may be doubled by administration of a doubled dose. At the end of a 3-hour i.v. infusion of cloxacillin (Cloxapen, Cloxacap and Orbenin) 250 mg given to normal subjects, its plasma concentrations were 15 ug/ml. After 2 hours, plasma concentrations were 0.6 ug/ml [1]. |
| Name | cloxacillin sodium monohydrate |
|---|---|
| Synonym | More Synonyms |
| Description | Cloxacillin sodium monohydrate is a semi-synthetic antibiotic that is a chlorinated derivative of oxacillin.Target: AntibacterialCloxacillin sodium (Cloxacap) is a sodium salt of cloxacillin that is a penicillinase-resistant, acid resistant, semi-synthetic penicillin. Cloxacillin sodium exerts a bactericidal action against susceptible microorganisms during the stage of active multiplication. Cloxacillin sodium acts through the inhibition of biosynthesis of cell wall mucopeptides. Cloxacillin sodium is readily absorbed following i.m. administration and rapidly reaches therapeutically effective blood levels. Serum levels are approximately proportional to dosage. Peak plasma concentrations of 15 ug/ml have been observed 30 minutes after an i.m. injection of cloxacillin (Cloxapen, Cloxacap and Orbenin) 500 mg; plasma concentrations may be doubled by administration of a doubled dose. At the end of a 3-hour i.v. infusion of cloxacillin (Cloxapen, Cloxacap and Orbenin) 250 mg given to normal subjects, its plasma concentrations were 15 ug/ml. After 2 hours, plasma concentrations were 0.6 ug/ml [1]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 689.7ºC at 760 mmHg |
|---|---|
| Molecular Formula | C19H19ClN3NaO6S |
| Molecular Weight | 475.88 |
| Flash Point | 370.9ºC |
| PSA | 136.54000 |
| LogP | 0.99210 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H317-H319-H334-H335 |
| Precautionary Statements | P261-P280-P284-P304 + P340-P305 + P351 + P338-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | XH8920000 |
| HS Code | 2941109900 |
| HS Code | 2941109900 |
|---|
|
Combined disc methods for the detection of KPC- and/or VIM-positive Klebsiella pneumoniae: improving reliability for the double carbapenemase producers.
Clin. Microbiol. Infect. 19(9) , E412-5, (2013) Klebsiella pneumoniae strains co-producing klebsiella pneumoniae carbapenemase (KPC) and verona integron-encoded metallo-beta-lactamase (VIM) are frequently isolated in Greece and have also occurred i... |
|
|
Intravenous infusion of electrolyte solution changes pharmacokinetics of drugs: pharmacokinetics of ampicillin.
J. Vet. Pharmacol. Ther. 37(5) , 445-50, (2014) The pharmacokinetics of ampicillin in dogs was determined after intravenous (i.v.) bolus and constant rate infusion. Ampicillin was administered to six beagle dogs as an i.v. bolus at 20 mg/kg and as ... |
|
|
Opalescent grouped vesicles over the face: an important indicator of staphylococcal septicemia.
Pediatr. Dermatol. 29(2) , 230-2, (2012) We present a report of three cases with vesicles containing opalescent fluid grouped over the face and scattered on the trunk and limbs. Culture of the fluid aspirated from the vesicles grew Staphyloc... |
| Orbenin |
| Tegopen |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[[3-(2-chlorophenyl)-5-methyl-4-isoxazolyl]carbonyl]amino]-3,3-dimethyl-7-oxo-, sodium salt, (2S,5R,6R)-, hydrate (1:1:1) |
| Staphybiotic |
| Cloxacillin Sodium Salt Monohydrate |
| cloxacillin sodium hydrate |
| Sodium (2S,5R,6R)-6-({[3-(2-chlorophenyl)-5-methyl-1,2-oxazol-4-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate hydrate (1:1:1) |
| MFCD00150735 |
| Ankerbin |
| Cloxapen |
| Prostaphilin A |
| Austrastaph |
| Ekvacillin |
| (2S,5R,6R)-6-[[[3-(2-Chlorophenyl)-5-methyl-4-isoxazolyl]carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid Sodium Salt Monohydrate |
| [(2S,5R,6R)-6-({[3-(2-Chlorophenyl)-5-methyl-1,2-oxazol-4-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylato-κO]sodium hydrate (1:1) |
| Prevencilina P |
| Cloxacillin sodium salt hydrate |
| sodium, [(2S,5R,6R)-6-[[[3-(2-chlorophenyl)-5-methyl-4-isoxazolyl]carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylato-κO]-, hydrate (1:1) |
| EINECS 211-390-9 |
| Gelstaph |
| Monosodium (2S,5R,6R)-6-(3-(o-Chlorophenyl)-5-methyl-4-isoxazolecarboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate Monohydrate |
| Sodium cloxacillin monohydrate |
| Cloxacillin Sodium |
| cloxacillin sodium monohydrate |
| Cloxacillin (sodium monohydrate) |

