Bay K-8644
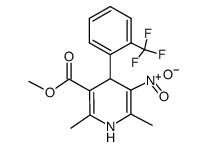
Bay K-8644 structure
|
Common Name | Bay K-8644 | ||
|---|---|---|---|---|
| CAS Number | 71145-03-4 | Molecular Weight | 356.29700 | |
| Density | 1.37g/cm3 | Boiling Point | 404.3ºC at 760 mmHg | |
| Molecular Formula | C16H15F3N2O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 198.3ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Bay K-8644Bay K 8644, a dihydropyridine compound, is a specific L-type Ca2+ channel agonist. Bay K 8644 increases Ca2+ influx through sarcolemmal Ca2+ channels by increasing the open time of the channel[1]. |
| Name | 1,4-Dihydro-2,6-dimethyl-3-nitro-4-(2-trifluoromethylphenyl)-pyridine-5-carboxylic acid methyl ester |
|---|---|
| Synonym | More Synonyms |
| Description | Bay K 8644, a dihydropyridine compound, is a specific L-type Ca2+ channel agonist. Bay K 8644 increases Ca2+ influx through sarcolemmal Ca2+ channels by increasing the open time of the channel[1]. |
|---|---|
| Related Catalog | |
| In Vitro | In newborn rat ventricular cardiomyocytes, Bay K 8644 (1 μM) treatment increases L-type calcium current density in 2-day-old cells. The higher increase of L-type calcium current density by Bay K 8644 in 2-day- than in 7-day-old cultured cells could be interpreted as the result of a difference in the phosphorylation level of calcium channels for each stage of development[2]. |
| In Vivo | A one time dose as low as 10 μg/kg of Bay K 8644 significantly elevates mean arterial pressure (MAP) in endotoxin-treated hypotensive rats while having minimal effects in normal rats. Bay K 8644 also causes a dose-dependent decrease in heart rate of 37% in endotoxin-treated rats and 39% in control rats[1]. |
| References |
| Density | 1.37g/cm3 |
|---|---|
| Boiling Point | 404.3ºC at 760 mmHg |
| Molecular Formula | C16H15F3N2O4 |
| Molecular Weight | 356.29700 |
| Flash Point | 198.3ºC |
| Exact Mass | 356.09800 |
| PSA | 84.15000 |
| LogP | 4.19940 |
| Index of Refraction | 1.545 |
| Storage condition | -20℃ |
|
~37% 
Bay K-8644 CAS#:71145-03-4 |
| Literature: Bayer Aktiengesellschaft Patent: US4532248 A1, 1985 ; |
|
~33% 
Bay K-8644 CAS#:71145-03-4 |
| Literature: Gorlitzer; Schmidt Archiv der Pharmazie, 1991 , vol. 324, # 10 p. 785 - 796 |
|
~21%
Detail
|
| Literature: Gorlitzer; Schmidt Archiv der Pharmazie, 1991 , vol. 324, # 10 p. 785 - 796 |
|
Metformin as adjunct antituberculosis therapy.
Sci. Transl. Med. 6(263) , 263ra159, (2014) The global burden of tuberculosis (TB) morbidity and mortality remains immense. A potential new approach to TB therapy is to augment protective host immune responses. We report that the antidiabetic d... |
|
|
Magnesium sulfate provides neuroprotection in lipopolysaccharide-activated primary microglia by inhibiting NF-κB pathway.
J. Surg. Res. 184(2) , 944-50, (2013) Magnesium sulfate has been used as an anticonvulsant in severe preeclamptic or eclamptic women prior to surgical trauma, but its effects on neuroinflammation is not well defined. In the present study,... |
|
|
Activity-dependent regulation of tyrosine hydroxylase expression in the enteric nervous system.
J. Physiol. 586(7) , 1963-75, (2008) The regulation of neuromediator expression by neuronal activity in the enteric nervous system (ENS) is currently unknown. Using primary cultures of ENS derived from rat embryonic intestine, we have ch... |
| (1,4-dihydro-2,6-dimethyl-5-nitro-4-[2-(trifluoromethyl)-phenyl]-3-pyridine carboxylic acid methyl ester |
| rac-Aziridin-2-carbonitril |
| methyl-1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-trifluoromethylphenyl)-pyridine-3-carboxylate |
| (+/-)-Aziridin-2-carbonitril |
| cyano-2 aziridine |
| (-)-2,6-dimethyl-3-carbomethoxy-5-nitro-4-(2-trifluoromethylphenyl)-1,4-dihydropyridine |
| 2-aziridinecarbonitrile |
| MFCD00036697 |
| 2-cyano-2-aziridine |
| (.+/-.)-Bay K 8644 |
| nitrile of aziridine-2-carboxylic acid |
| Bay K-8644 |



![1,4-Dihydro-2,6-dimethyl-3,5-dinitro-4-[2-(trifluormethyl)phenyl]-pyridin structure](https://image.chemsrc.com/caspic/378/137481-69-7.png)