Nafcillin sodium salt monohydrate
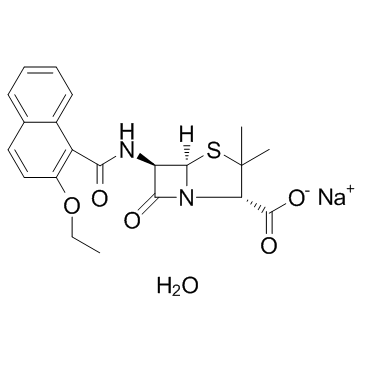
Nafcillin sodium salt monohydrate structure
|
Common Name | Nafcillin sodium salt monohydrate | ||
|---|---|---|---|---|
| CAS Number | 7177-50-6 | Molecular Weight | 454.472 | |
| Density | 1.42 g/cm3 | Boiling Point | 714.1ºC at 760 mmHg | |
| Molecular Formula | C21H23N2NaO6S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 385.7ºC | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of Nafcillin sodium salt monohydrateNafcillin sodium monohydrate is a semi-synthetic antibiotic related to penicillin.Target: AntibacterialNafcillin sodium is a narrow-spectrum, beta-lactam antibiotic of the penicillin class. As a beta-lactamase-resistant penicillin, it is used to treat infections caused by Gram-positive bacteria, in particular, species of staphylococci that are resistant to other penicillins. Nafcillin is considered therapeutically equivalent to oxacillin, although its safety profile is somewhat different. Nafcillin was shown to reversibly inhibit beta-lactamase from Staphylococcus aureus PC1 with characteristics indicative of a type A inhibitor [Citri, Samuni & Zyk (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 1048-1052]. At nafcillin concentrations above 80 mM, complete inactivation occurred within 200 s. Upon removal of the excess nafcillin the inhibited enzyme was re-activated completely, with a rate constant of 2.0 x 10(-3) s-1 (25 degrees C) [1, 2]. |
| Name | nafcillin sodium monohydrate |
|---|---|
| Synonym | More Synonyms |
| Description | Nafcillin sodium monohydrate is a semi-synthetic antibiotic related to penicillin.Target: AntibacterialNafcillin sodium is a narrow-spectrum, beta-lactam antibiotic of the penicillin class. As a beta-lactamase-resistant penicillin, it is used to treat infections caused by Gram-positive bacteria, in particular, species of staphylococci that are resistant to other penicillins. Nafcillin is considered therapeutically equivalent to oxacillin, although its safety profile is somewhat different. Nafcillin was shown to reversibly inhibit beta-lactamase from Staphylococcus aureus PC1 with characteristics indicative of a type A inhibitor [Citri, Samuni & Zyk (1976) Proc. Natl. Acad. Sci. U.S.A. 73, 1048-1052]. At nafcillin concentrations above 80 mM, complete inactivation occurred within 200 s. Upon removal of the excess nafcillin the inhibited enzyme was re-activated completely, with a rate constant of 2.0 x 10(-3) s-1 (25 degrees C) [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.42 g/cm3 |
|---|---|
| Boiling Point | 714.1ºC at 760 mmHg |
| Molecular Formula | C21H23N2NaO6S |
| Molecular Weight | 454.472 |
| Flash Point | 385.7ºC |
| Exact Mass | 454.117462 |
| PSA | 133.30000 |
| LogP | 1.41360 |
| Storage condition | 2-8°C |
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H317-H319-H334-H335 |
| Precautionary Statements | P261-P280-P284-P304 + P340-P305 + P351 + P338-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Effect of NaCl and nafcillin on penicillin-binding protein 2a and heterogeneous expression of methicillin resistance in Staphylococcus aureus.
Antimicrob. Agents Chemother. 31 , 1982-1988, (1987) Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus is enhanced by 2 to 5% NaCl in the medium and by selection with beta-lactam antibiotics. Resistance is associated... |
|
|
Co-trimoxazole versus nafcillin in the therapy of experimental meningitis due to Staphylococcus aureus.
J. Antimicrob. Chemother. 19 , 647-658, (1987) Co-trimoxazole was compared with nafcillin against Staphylococcus aureus in vitro and in the therapy of experimental Staph. aureus meningitis in rabbits. Co-trimoxazole (trimethoprim:sulphamethoxazole... |
| nafcillin sodium monohydrate |
| MFCD01941128 |
| Nafcillin sodium salt monohydrate |
| NAFCILLINSODIUMSTERILE |
| nafcillin sodium |
| Unipen (tn) |
| Sodium (2S,5R,6R)-6-[(2-ethoxy-1-naphthoyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate hydrate (1:1:1) |
| Nafcillin monohydrate sodium salt |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[(2-ethoxy-1-naphthalenyl)carbonyl]amino]-3,3-dimethyl-7-oxo-, sodium salt, (2S,5R,6R)-, hydrate (1:1:1) |
| Nafcillin (sodium monohydrate) |