Dihydroartemisinin

Dihydroartemisinin structure
|
Common Name | Dihydroartemisinin | ||
|---|---|---|---|---|
| CAS Number | 71939-50-9 | Molecular Weight | 284.348 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 375.6±42.0 °C at 760 mmHg | |
| Molecular Formula | C15H24O5 | Melting Point | 144-149ºC | |
| MSDS | N/A | Flash Point | 181.0±27.9 °C | |
Use of DihydroartemisininDihydroartemisinin is a potent anti-malaria agent. |
| Name | Dihydroartemisinin |
|---|---|
| Synonym | More Synonyms |
| Description | Dihydroartemisinin is a potent anti-malaria agent. |
|---|---|
| Related Catalog | |
| Target |
RelA Autophagy |
| In Vitro | Dihydroartemisinin (DHA) is an antimalarial agent. Dihydroartemisinin treatment effectively up-regulates the cytosolic RelA/p65 protein level and down-regulates the nuclear RelA/p65 protein level. Dihydroartemisinin blocks the nuclear translocation of RelA/p65 from the cytosol rather than suppressing RelA/p65 protein synthesis. Dihydroartemisinin induces autophagy in RPMI 8226 cells. Dihydroartemisinin suppresses NF-κB activation in RPMI 8226 cells. The NF-κB Dihydroartemisinin -binding activity is examined by EMSA assay. RPMI 8226 cells are exposed to various concentrations of Dihydroartemisinin (10, 20 and 40 μM) for 12 h, and TNF-α is introduced as a positive control for NF-κB activation. Dihydroartemisinin suppresses NF-κB activation in a dose-dependent manner in contrast with TNF-α[1]. Dihydroartemisinin (DHA) can enhance the anti-tumor effect of photodynamic therapy (PDT) on esophageal cancer cells, and cell viability is investigated using the MTT assay. Eca109 and Ec9706 cells are treated with Dihydroartemisinin (80 μM), PDT (25 and 20 J/cm2, respectively) or their combination. Single treatment with Dihydroartemisinin or PDT causes a 37±5% or 34±6% reduction in viability in Eca109 cells and a 33±7% or 34±6% reduction in Ec9706 cells, respectively. However, when PDT is combined with Dihydroartemisinin, the cell viability is reduced 59±6% or 61±7% in the cell lines, respectively[2]. |
| In Vivo | Single oral doses of Dihydroartemisinin (at 200, 300, 400 or 600 mg/kg), given once on each of day 6-8 post-infection, reduce total-worm burdens by 69.2%-90.6% and female-worm burdens by 62.2%-92.2%, depending on dosage in the first experiment. Similar treatments given on day 34-36 post-infection reduce total-worm burdens by 73.9%-85.5% and female-worm burdens by 83.8%-95.3%[3]. |
| Kinase Assay | To determine NF-κB Dihydroartemisinin-binding activity, an electrophoretic mobility shift assay (EMSA) is performed. Nuclear extracts are prepared and incubated with 32P-end-labeled 45-mer double-stranded oligonucleotide (15 μg protein with 16 fmol DNA) from the HIV long terminal repeat, 5′-TTGTTACAAGGGACTTTCCGCTG GGGACTTTCCAGGGAGGCGTGG-3′ (boldface indicates NF-κB binding sites), for 30 min at 37 °C. The Dihydroartemisinin-protein complex formed is separated from free oligonucleotide on 6.6% native polyacrylamide gels. A double-stranded mutated oligonucleotide, 5′-TTGTTACAA CTCACTTTCCGCTGCTCACTTTCCAGGGAGGCGTGG-3′, is used to examine binding specificity of NF-κB to the DNA. The binding specificity is also examined by competition with the unlabeled oligonucleotide. Preimmune serum (PIS) is included as a negative control. The dried gels are visualized with a Storm 820, and radioactive bands are quantified using Imagequant software[1]. |
| Cell Assay | Eca109 (4×103 cells/well) and Ec9706 (5×103 cells/well) cells are grown in 96-well plates and cultured overnight to allow for cell attachment. Eca109 and Ec9706 cells are treated with Dihydroartemisinin (80 μM), PDT (25 and 20 J/cm2, respectively) or their combination. After incubation for 24h, MTT (20 μL) is added to each well and incubated for 4 h at 37°C. Formazan crystals are dissolved in 150 μL of DMSO for 10 min with shaking. The absorbance is measured at 490 nm on a plate reader, and the experiment is repeated three times[2]. |
| Animal Admin | Mice[3] Mice of the Kunming strain, each weighing 20-24 g, are used. In the first experiment, design to investigate the effect of multiple doses of Dihydroartemisinin on the schistosomula and adult worms of S. japonicum, mice are given three daily doses, of 200, 300, 400 or 600 mg Dihydroartemisinin/kg (in dose volumes of 25 mL/kg), on days 6-8 or 34-36 post-infection, respectively. An additional group of mice, infected but not given the drug, serve as a control. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 375.6±42.0 °C at 760 mmHg |
| Melting Point | 144-149ºC |
| Molecular Formula | C15H24O5 |
| Molecular Weight | 284.348 |
| Flash Point | 181.0±27.9 °C |
| Exact Mass | 284.162384 |
| PSA | 57.15000 |
| LogP | 2.27 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.543 |
| Storage condition | 2-8°C |
|
~98% 
Dihydroartemisinin CAS#:71939-50-9 |
| Literature: Avery, Mitchell A.; Mehrotra, Sanjiv; Johnson, Theresa L.; Bonk, Jason D.; Vroman, Jeffrey A.; Miller, Robert Journal of Medicinal Chemistry, 1996 , vol. 39, # 21 p. 4149 - 4155 |
|
~% 
Dihydroartemisinin CAS#:71939-50-9 |
| Literature: Organic Process Research and Development, , vol. 16, # 5 p. 1039 - 1042 |
|
~% 
Dihydroartemisinin CAS#:71939-50-9 |
| Literature: Molecules, , vol. 15, # 12 p. 8747 - 8768 |
| Precursor 2 | |
|---|---|
| DownStream 10 | |
| Dihydroartemisinin |
| Dihydroartemisin |
| 3,12-Epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10-ol, decahydro-3,6,9-trimethyl-, (3R,5aS,6R,8aS,9R,10S,12R,12aR)- |
| Cotecxin |
| Dihydroginghaosu |
| (1S,4S,7R,8S,11R,12S,13R)-1,7,11-Trimethyl-3,5,14,15-tetraoxatetracyclo[10.3.1.0.0]hexadecan-6-ol |
| MFCD00274495 |
| (1R,4S,5R,8S,9R,10S,12R,13R)-1,5,9-Trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.0.0]hexadecan-10-ol |
| Alaxin |
| [3H]-(10R/S)-Artenimol |
| Salaxin |
| artenimol |
| 3,12-Epoxy-12H-pyrano(4,3-j)-1,2-benzodioxepin-10-ol, decahydro-3,6,9-trimethyl-, (3R,5aS,6R,8aS,9R,10S,12R,12aR)- |
| (3R,5aS,6R,8aS,9R,12S,12aR)-decahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10-ol |
| (3R,5aS,6R,8aS,9R,12R,12aR)-decahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10-ol |
| dihyhydroartemisinin |
| DHQHS 2 |
| (5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-trimethyldecahydro-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10-ol |
| 3,12-Methano-5aH,7H-1,2,5-trioxepino[3,4-j][2]benzopyran-7-ol, octahydro-3,8,11-trimethyl-, (3S,5aS,8R,8aS,11R,12S,12aR)- |
| Dihydroarteminisin |
| dihydroquinghaosu |
| (3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-trimethyldecahydro-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10-ol |
| Cotexin |
| Dihydroqinghaosu |

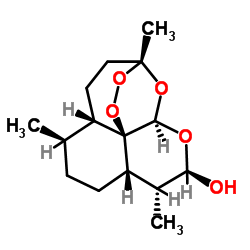
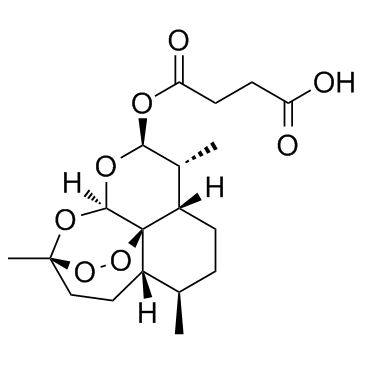 CAS#:88495-63-0
CAS#:88495-63-0![(3R,12aR)-3,6α,9β-Trimethyl-3β,12α-epoxy-3,4,5,5aα,6,7,8,8aα,9,10-decahydro-10α-ethoxypyrano[4,3-j]-1,2-benzodioxepin structure](https://image.chemsrc.com/caspic/076/82534-75-6.png) CAS#:82534-75-6
CAS#:82534-75-6 CAS#:75887-54-6
CAS#:75887-54-6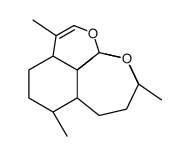 CAS#:82596-30-3
CAS#:82596-30-3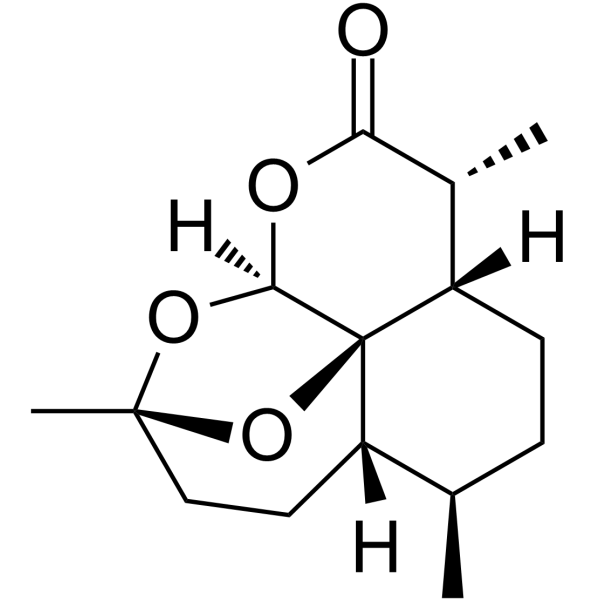 CAS#:72826-63-2
CAS#:72826-63-2![(2S,3R,6R)-2-(3-oxobutyl)-3-methyl-6-[(R)2-propanal]-cyclohexanone structure](https://image.chemsrc.com/caspic/368/107466-89-7.png) CAS#:107466-89-7
CAS#:107466-89-7![(2S,3R,6S)-2-(3-oxobutyl)-3-methyl-6-[(R)2-propanal]-cyclohexanone structure](https://image.chemsrc.com/caspic/406/107466-88-6.png) CAS#:107466-88-6
CAS#:107466-88-6![(2S,3R,6RS)-2-(3-Oxobutyl)-3-Methyl-6-[(R)-2-propanal]cyclohexanone structure](https://image.chemsrc.com/caspic/080/1093625-96-7.png) CAS#:1093625-96-7
CAS#:1093625-96-7 CAS#:71963-77-4
CAS#:71963-77-4
