Fmoc-Ile-OH
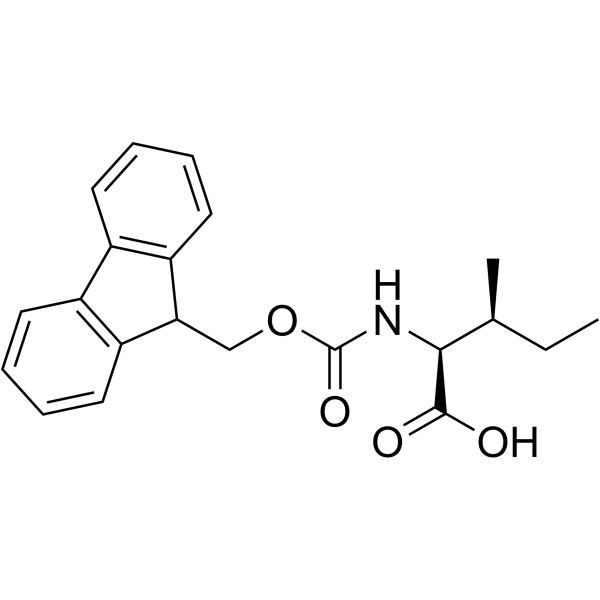
Fmoc-Ile-OH structure
|
Common Name | Fmoc-Ile-OH | ||
|---|---|---|---|---|
| CAS Number | 71989-23-6 | Molecular Weight | 353.412 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 559.8±33.0 °C at 760 mmHg | |
| Molecular Formula | C21H23NO4 | Melting Point | 145-147 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 292.4±25.4 °C | |
Use of Fmoc-Ile-OHFmoc-Ile-OH is an isoleucine derivative[1]. |
| Name | Fmoc-L-Isoleucine |
|---|---|
| Synonym | More Synonyms |
| Description | Fmoc-Ile-OH is an isoleucine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 559.8±33.0 °C at 760 mmHg |
| Melting Point | 145-147 °C(lit.) |
| Molecular Formula | C21H23NO4 |
| Molecular Weight | 353.412 |
| Flash Point | 292.4±25.4 °C |
| Exact Mass | 353.162720 |
| PSA | 75.63000 |
| LogP | 4.95 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.583 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924299090 |
|
~89% 
Fmoc-Ile-OH CAS#:71989-23-6 |
| Literature: Raydnov, M. G.; Klimenko, L. V.; Mitin, Yu. V. Russian Journal of Bioorganic Chemistry, 1999 , vol. 25, # 5 p. 283 - 287 Bioorganicheskaya Khimiya, 1999 , vol. 25, # 5 p. 323 - 328 |
|
~69% 
Fmoc-Ile-OH CAS#:71989-23-6 |
| Literature: Chinchilla, Rafael; Dodsworth, David; Najera, Carmen; Soriano, Jose Tetrahedron Letters, 2001 , vol. 42, # 43 p. 7579 - 7581 |
|
~82% 
Fmoc-Ile-OH CAS#:71989-23-6 |
| Literature: Schoen, Istvan; Kisfaludy, Lajos Synthesis, 1986 , # 4 p. 303 - 305 |
|
~81% 
Fmoc-Ile-OH CAS#:71989-23-6 |
| Literature: Echner, Hartmut; Voelter, Wolfgang Liebigs Annalen der Chemie, 1988 , p. 1095 - 1098 |
|
~% 
Fmoc-Ile-OH CAS#:71989-23-6 |
| Literature: Journal of Organic Chemistry, , vol. 77, # 23 p. 10575 - 10582 |
|
~% 
Fmoc-Ile-OH CAS#:71989-23-6 |
| Literature: Journal of the Chinese Chemical Society, , vol. 58, # 4 p. 509 - 515 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Complete ON/OFF photoswitching of the motility of a nanobiomolecular machine.
ACS Nano 8(5) , 4157-65, (2014) To apply motor proteins as natural nanomolecular machines to transporting systems in nanotechnology, complete temporal control over ON/OFF switching of the motility is necessary. We have studied the p... |
|
|
Design and synthesis of newN-(fluorenyl-9-methoxycarbonyl) (Fmoc)-dipeptides as anti-inflammatory agents
Eur. J. Med. Chem. 44 , 1933-40, (2009) Twenty-four new dipeptide analogs ( 1– 24) of aurantiamide acetate were designed, synthesized, and assayed for effects on superoxide anion generation and elastase release by human neutrophils in respo... |
| L-Isoleucine, N-[(9H-fluoren-9-ylmethoxy)carbonyl]- |
| N-[(9H-Fluoren-9-ylmethoxy)carbonyl]-L-isoleucine |
| N-((9H-Fluoren-9-ylmethoxy)carbonyl)-L-isoleucine |
| EINECS 276-255-9 |
| MFCD00037125 |
| N-(9-Fluorenylmethoxycarbonyl)-L-isoleucine |
| N-Fmoc-Ile-OH ( N-Fmoc-L-isoleucine) |
| FMOC-L-Isoleucine |
| Fmoc-Ile-OH |

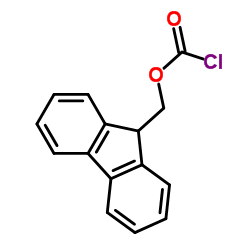

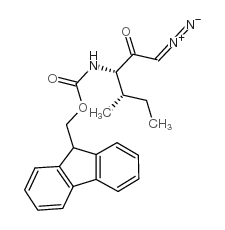 CAS#:266359-44-8
CAS#:266359-44-8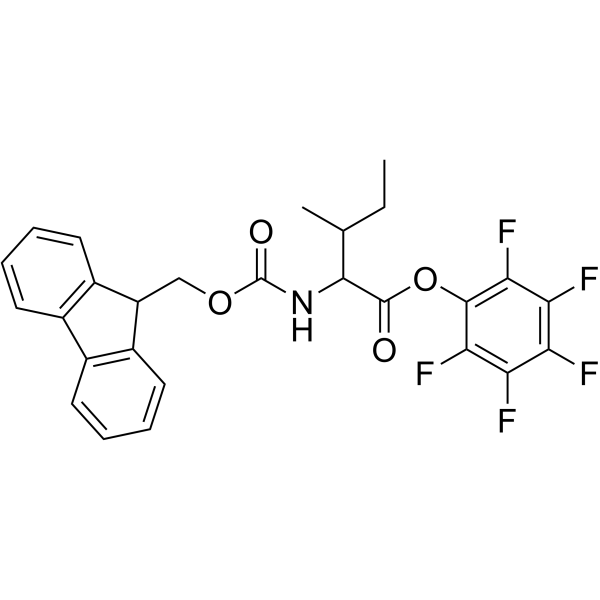 CAS#:86060-89-1
CAS#:86060-89-1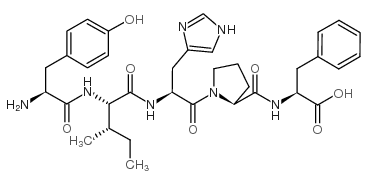 CAS#:52530-60-6
CAS#:52530-60-6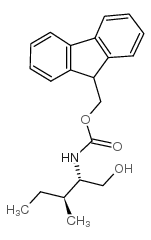 CAS#:133565-46-5
CAS#:133565-46-5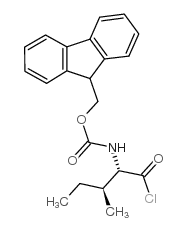 CAS#:103321-51-3
CAS#:103321-51-3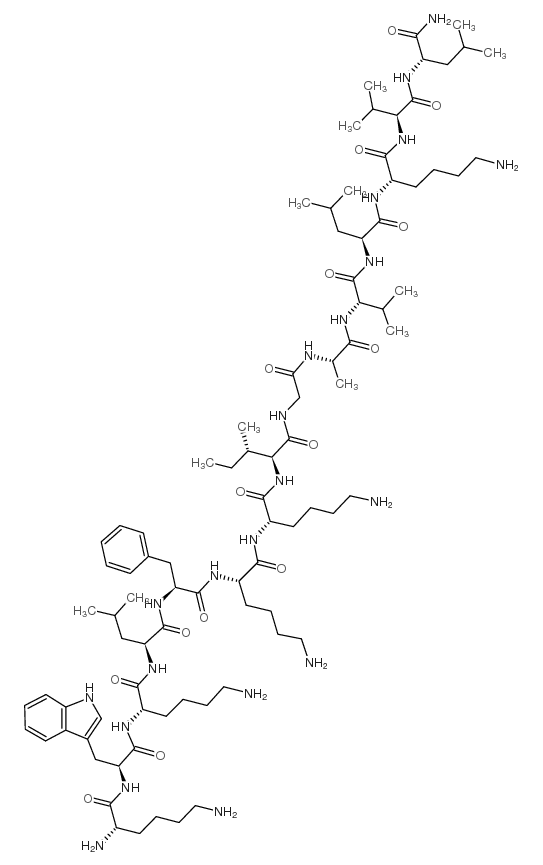 CAS#:157606-25-2
CAS#:157606-25-2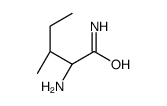 CAS#:14445-54-6
CAS#:14445-54-6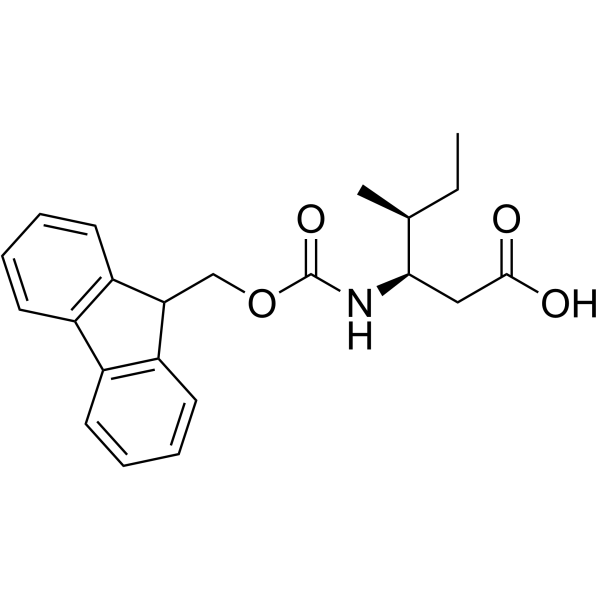 CAS#:193954-27-7
CAS#:193954-27-7 CAS#:121377-67-1
CAS#:121377-67-1
