4-(N-BOC-AMINO)PIPERIDINE
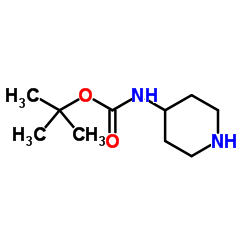
4-(N-BOC-AMINO)PIPERIDINE structure
|
Common Name | 4-(N-BOC-AMINO)PIPERIDINE | ||
|---|---|---|---|---|
| CAS Number | 73874-95-0 | Molecular Weight | 200.28 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 304.8±31.0 °C at 760 mmHg | |
| Molecular Formula | C10H20N2O2 | Melting Point | 162-166 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 138.2±24.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 4-(N-BOC-AMINO)PIPERIDINE4-Boc-aminopiperidine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 4-Boc-aminopiperidine |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Boc-aminopiperidine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 4-(N-Boc-amino)piperidine 是一种有机结构单元。它已被用于合成氨基哌啶抗病毒趋化因子 (CC motif) 受体 5 (CCR5) 拮抗剂和抗菌药物。 |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 304.8±31.0 °C at 760 mmHg |
| Melting Point | 162-166 °C(lit.) |
| Molecular Formula | C10H20N2O2 |
| Molecular Weight | 200.28 |
| Flash Point | 138.2±24.8 °C |
| Exact Mass | 200.152481 |
| PSA | 50.36000 |
| LogP | 1.32 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.480 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933399090 |
|
~98% 
4-(N-BOC-AMINO)... CAS#:73874-95-0 |
| Literature: KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY; CRYSTALGENOMICS, INC. Patent: WO2007/52938 A1, 2007 ; Location in patent: Page/Page column 18 ; WO 2007/052938 A1 |
|
~% 
4-(N-BOC-AMINO)... CAS#:73874-95-0 |
| Literature: US2003/162772 A1, ; |
|
~% 
4-(N-BOC-AMINO)... CAS#:73874-95-0 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 18 p. 2475 - 2479 |
|
~% 
4-(N-BOC-AMINO)... CAS#:73874-95-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 49, # 6 p. 788 - 790 |
|
~% 
4-(N-BOC-AMINO)... CAS#:73874-95-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 42, # 14 p. 2706 - 2715 |
|
~% 
4-(N-BOC-AMINO)... CAS#:73874-95-0 |
| Literature: US2001/51727 A1, ; US 20010051727 A1 |
|
~% 
4-(N-BOC-AMINO)... CAS#:73874-95-0 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 18 p. 2475 - 2479 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Discovery of a piperidine-4-carboxamide CCR5 antagonist (TAK-220) with highly potent Anti-HIV-1 activity.
J. Med. Chem. 49 , 2784, (2006) We incorporated various polar groups into previously described piperidine-4-carboxamide CCR5 antagonists to improve their metabolic stability in human hepatic microsomes. Introducing a carbamoyl group... |
|
|
Synthesis of potent oxindole CDK2 inhibitors.
Bioorg. Med. Chem. 11 , 1873-1881, (2003) A series of oxindole CDK2 inhibitors was synthesized. These novel analogues have a saturated monosubstituted cyclic moiety at their C-4 position that mimics the ribofuranoside of ATP. This substitutio... |
|
|
Poly (2-isopropyl-2-oxazoline)-poly (L-glutamate) block copolymers through ammonium-mediated NCA polymerization. Meyer M and Schlaad H.
Macromolecules 39(11) , 3967-3970, (2006)
|
| 2-Methyl-2-propanyl 4-amino-1-piperidinecarboxylate |
| Carbamic acid, N-4-piperidinyl-, 1,1-dimethylethyl ester |
| MFCD00798171 |
| methanol, 1-(1,1-dimethylethoxy)-1-(4-piperidinylimino)-, (E)- |
| tert-butyl N-piperidin-4-ylcarbamate |
| tert-butyl-piperidin-4-ylcarbamat |
| 2-Methyl-2-propanyl 4-piperidinylcarbamate |
| tert-butyl piperidin-4-ylcarbamate |
| 4-(N-BOC-AMINO)PIPERIDINE |
| 4-(tert-Butoxycarbonyl)-aminopiperidine |
| 4-N-BOC-Aminopiperidine |
| 4-N-(tert-Butoxycarbonyl)aminopiperidine |




 CAS#:110883-96-0
CAS#:110883-96-0 CAS#:402927-97-3
CAS#:402927-97-3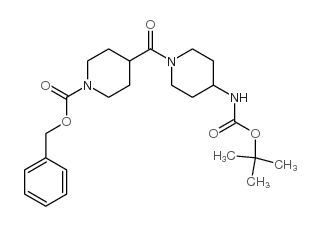 CAS#:220031-94-7
CAS#:220031-94-7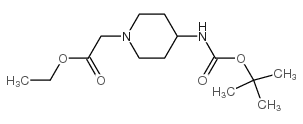 CAS#:203662-91-3
CAS#:203662-91-3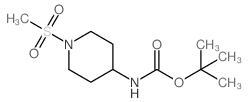 CAS#:287953-38-2
CAS#:287953-38-2![2-(4-([(TERT-BUTOXY)CARBONYL]AMINO)PIPERIDIN-1-YL)ACETIC ACID structure](https://image.chemsrc.com/caspic/496/299203-94-4.png) CAS#:299203-94-4
CAS#:299203-94-4![(5'-Methyl-3,4,5,6-tetrahydro-2H-[1,2']bipyridinyl-4-yl)-carbamic acid tert-butyl ester structure](https://image.chemsrc.com/caspic/020/252578-18-0.png) CAS#:252578-18-0
CAS#:252578-18-0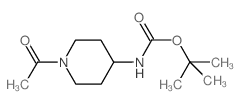 CAS#:283167-28-2
CAS#:283167-28-2 CAS#:85098-70-0
CAS#:85098-70-0 CAS#:741713-40-6
CAS#:741713-40-6
