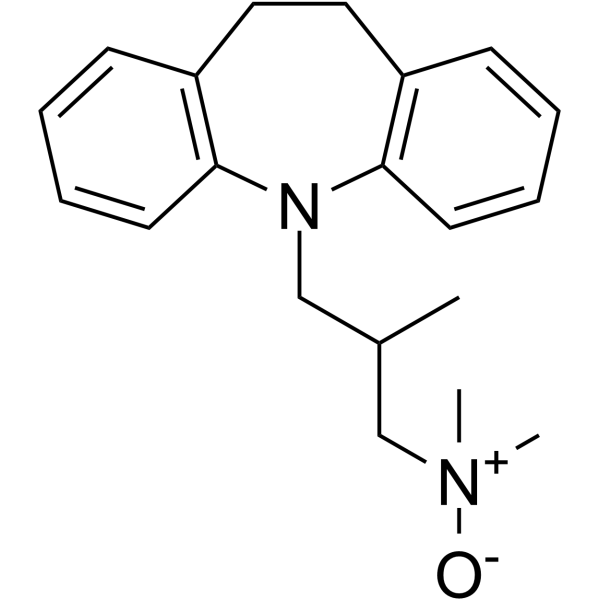
trimipramine
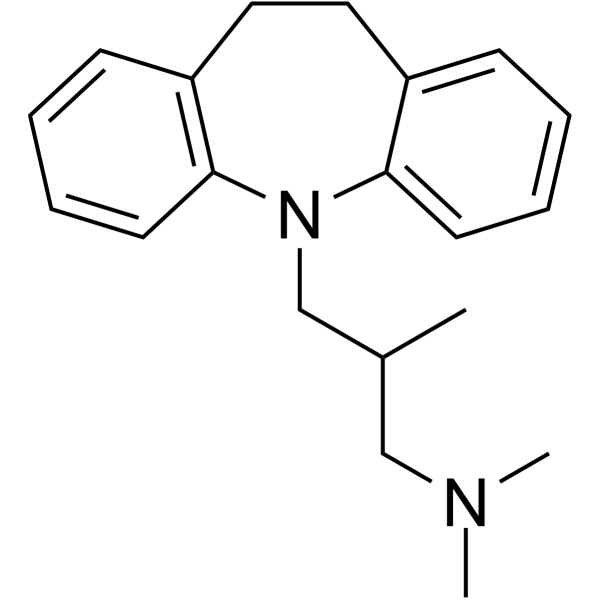
trimipramine structure
|
Common Name | trimipramine | ||
|---|---|---|---|---|
| CAS Number | 739-71-9 | Molecular Weight | 294.43400 | |
| Density | 0.9912 (rough estimate) | Boiling Point | 426.2°C (rough estimate) | |
| Molecular Formula | C20H26N2 | Melting Point | 45° | |
| MSDS | N/A | Flash Point | 9℃ | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of trimipramineTrimipramine is a 5-HT receptor antagonist, with pKi binding values of 6.39, 8.10, 4.66 for 5-HT1C, 5-HT2 and 5-HT1A, respectively. Trimipramine is also a potent and selective inhibitor targeting human noradrenaline (hNAT), serotonin (hSERT) and organic cation transporters (hOCT1, hOCT2) with IC50 values of 4.99 μM, 2.11 μM, 3.72 μM, 8.00 μM, respectively. Trimipramine has vascular activity and anxiolytic efficacy[1][2][3]. |
| Name | trimipramine |
|---|---|
| Synonym | More Synonyms |
| Description | Trimipramine is a 5-HT receptor antagonist, with pKi binding values of 6.39, 8.10, 4.66 for 5-HT1C, 5-HT2 and 5-HT1A, respectively. Trimipramine is also a potent and selective inhibitor targeting human noradrenaline (hNAT), serotonin (hSERT) and organic cation transporters (hOCT1, hOCT2) with IC50 values of 4.99 μM, 2.11 μM, 3.72 μM, 8.00 μM, respectively. Trimipramine has vascular activity and anxiolytic efficacy[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
5-HT1C Receptor:6.39 (pKi) 5-HT2 Receptor:8.10 (pKi) 5-HT1A Receptor:4.66 (pKi) |
| In Vitro | Trimipramine displays much higher affinity for 5-HT2 than for 5-HT1C receptors[1]. Trimipramine is a moderate inhibitor of the human NAT and SERT, with the IC50 values of 4.99 μM and 2.11 μM, respectively[2]. SERT and NAT could represent a target for the antidepressant effects of trimipramine (1 mM, 0.1 mM, 0.01 mM, 1 μM, 0.1 μM; 10 min; HEK293 cells)[2]. |
| In Vivo | Trimipramine (5 mg/kg/d; 14 d; chronic administration) acts as functions in rats:1. Increasing concentration of regional 5-HT. 5-HT is highest in the frontal cortex and the hippocampus, followed by the olfactory tubercles and the hypothalamus. 2. Decreasing the number of frontal cortex 5-HT2 and striatal DA D2 receptors. 3. Increasing in the brain regional level of monoamines and metabolites. thus indicates a greater synthesis rate for dopamine (DA) and 5-HT coinciding with an adaptive down regulation of 5-HT2 and DA D2 receptors[3]. Animal Model: Male Wistar rats (220-250 g); implanted osmotic minipump subcutaneously in the dorsal thoracic interscapular region[3] Dosage: 5 mg/kg/day Administration: Delivered by smotic minipump; 14 days Result: Decreased the number of frontal cortex 5-HT2 and striatal DA D2 receptors, thus blocked the uptake of 5-HT and dopamine (DA). |
| References |
| Density | 0.9912 (rough estimate) |
|---|---|
| Boiling Point | 426.2°C (rough estimate) |
| Melting Point | 45° |
| Molecular Formula | C20H26N2 |
| Molecular Weight | 294.43400 |
| Flash Point | 9℃ |
| Exact Mass | 294.21000 |
| PSA | 6.48000 |
| LogP | 4.18600 |
| Index of Refraction | 1.6450 (estimate) |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Hazard Codes | F,T |
| Risk Phrases | 11-23/24/25-39/23/24/25 |
| Safety Phrases | 16-36/37-45 |
| RIDADR | UN 3249 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
~92% 
trimipramine CAS#:739-71-9 |
| Literature: Gowda, Narendra B.; Rao, Gopal Krishna; Ramakrishna, Ramesha A. Tetrahedron Letters, 2010 , vol. 51, # 43 p. 5690 - 5693 |
|
~% 
trimipramine CAS#:739-71-9 |
| Literature: Somashekar; Nagana Gowda; Ramesha; Khetrapal Magnetic Resonance in Chemistry, 2005 , vol. 43, # 2 p. 166 - 170 |
|
Evaluation of the matrix effect of different sample matrices for 33 pharmaceuticals by post-column infusion.
J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 1000 , 84-94, (2015) Matrix effects that occur during quantitative measurement by liquid chromatography mass spectrometry specifically when using electrospray ionization are a widely recognized phenomenon. Sample matrix c... |
|
|
Antidepressants for patients with tinnitus.
Cochrane Database Syst. Rev. 9 , CD003853, (2012) This is an update of a Cochrane review first published in The Cochrane Library in Issue 4, 2006 and previously updated in 2009.Tinnitus is described as the perception of sound or noise in the absence ... |
|
|
Seizures after single-agent overdose with pharmaceutical drugs: analysis of cases reported to a poison center.
Clin. Toxicol. (Phila.) 52(6) , 629-34, (2014) Seizures during intoxications with pharmaceuticals are a well-known complication. However, only a few studies report on drugs commonly involved and calculate the seizure potential of these drugs.To id... |
| (+-)-1-cyclohexyl-1-phenyl-3-piperidino-propan-1-ol,hydrochloride |
| (+-)-1-Cyclohexyl-1-phenyl-3-piperidino-propan-1-ol,Hydrochlorid |
| tremin |
| (+/-)-Trihexyphenidyl hydrochloride |
| EINECS 212-008-3 |
| Trihexy |
| 5-(3-dimethylamino-2-methylpropyl)-10,11-dihydro-5H-dibenz (b,f) azepine acid maleate |
| triiodothyronine |
| Trimipramine |
| paralest |
| 8-Chloro-3-methoxy-11-(1-methyl-4-piperidinyl)-6,11-dihydro-5H-benzo[5,6]-cyclohepta[1,2-b]pyridin-11-ol |
| [3-(10,11-dihydro-dibenzo[b,f]azepin-5-yl)-2-methyl-propyl]-dimethyl-amine |
| 10,11-dihydro-5-(3-dimethylamino-2-methylpropyl)-5H-dibenz(b,f)azepine |
| peragit |
| Sedrina |
| timipramine |
| cyclodol |
| trihexylphenidyl HCl |
| pacitane |
| Broflex |
| 3,3',5-triiodothyronine |
| L-3,3',5-triiodothyronine |
| 3,5,3'-triiodothyronine |
| pipanol |