Cilostazol
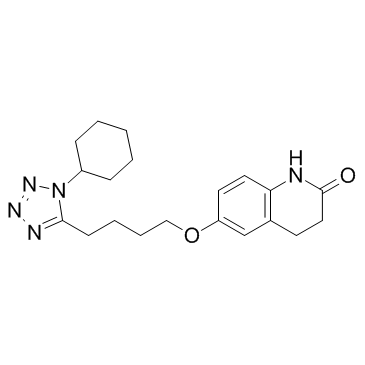
Cilostazol structure
|
Common Name | Cilostazol | ||
|---|---|---|---|---|
| CAS Number | 73963-72-1 | Molecular Weight | 369.461 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 664.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C20H27N5O2 | Melting Point | 159-160ºC | |
| MSDS | Chinese USA | Flash Point | 355.8±31.5 °C | |
Use of CilostazolCilostazol(OPC 13013; OPC 21) is a potent inhibitor of PDE3A, the isoform of PDE 3 in the cardiovascular system (IC50=0.2 uM).IC50 Value: 0.2 uM [1]Target: PDE3Ain vitro: Cilostazol caused a concentration-dependent increase in the cAMP level in rabbit and human platelets with similar potency. Furthermore, cilostazol and milrinone were equally effective in inhibiting human platelet aggregation with a median inhibitory concentration (IC50) of 0.9 and 2 microM, respectively. In rabbit ventricular myocytes, however, cilostazol elevated cAMP levels to a significantly lesser extent (p < 0.05 vs. milrinone) [2]. Cilostazol inhibited SIPA dose-dependently in vitro. The IC50 value of cilostazol for inhibition of SIPA was 15 +/- 2.6 microM (m +/- SE, n=5), which was very similar to that (12.5 +/- 2.1 microM) for inhibition of ADP-induced platelet aggregation. Cilostazolpotentiates the inhibition of SIPA by PGE1 and enhances its ability to increase cAMP concentrations [3].in vivo: A single oral adminstration of 100 mgcilostazol to healthy volunteers produced a significant inhibition of SIPA [3]. Male C57BL/6J mice were assigned to five groups: mice fed a normal diet (groups 1 and 2); 0.1% or 0.3% cilostazol-containing diet (groups 3 and 4, respectively); and 0.125% clopidogrel-containing diet (group 5). Two weeks after feeding, groups 2-5 were intraperitoneally administered carbon tetrachloride (CCl4 ) twice a week for 6 weeks, while group 1 was treated with the vehicle alone [4].Toxicity: Cilostazol in addition to dual antiplatelet therapy appears to be effective in reducing the risk of restenosis and repeat revascularization after PCI without any significant benefits for mortality or stent thrombosis [5]. |
| Name | cilostazol |
|---|---|
| Synonym | More Synonyms |
| Description | Cilostazol(OPC 13013; OPC 21) is a potent inhibitor of PDE3A, the isoform of PDE 3 in the cardiovascular system (IC50=0.2 uM).IC50 Value: 0.2 uM [1]Target: PDE3Ain vitro: Cilostazol caused a concentration-dependent increase in the cAMP level in rabbit and human platelets with similar potency. Furthermore, cilostazol and milrinone were equally effective in inhibiting human platelet aggregation with a median inhibitory concentration (IC50) of 0.9 and 2 microM, respectively. In rabbit ventricular myocytes, however, cilostazol elevated cAMP levels to a significantly lesser extent (p < 0.05 vs. milrinone) [2]. Cilostazol inhibited SIPA dose-dependently in vitro. The IC50 value of cilostazol for inhibition of SIPA was 15 +/- 2.6 microM (m +/- SE, n=5), which was very similar to that (12.5 +/- 2.1 microM) for inhibition of ADP-induced platelet aggregation. Cilostazolpotentiates the inhibition of SIPA by PGE1 and enhances its ability to increase cAMP concentrations [3].in vivo: A single oral adminstration of 100 mgcilostazol to healthy volunteers produced a significant inhibition of SIPA [3]. Male C57BL/6J mice were assigned to five groups: mice fed a normal diet (groups 1 and 2); 0.1% or 0.3% cilostazol-containing diet (groups 3 and 4, respectively); and 0.125% clopidogrel-containing diet (group 5). Two weeks after feeding, groups 2-5 were intraperitoneally administered carbon tetrachloride (CCl4 ) twice a week for 6 weeks, while group 1 was treated with the vehicle alone [4].Toxicity: Cilostazol in addition to dual antiplatelet therapy appears to be effective in reducing the risk of restenosis and repeat revascularization after PCI without any significant benefits for mortality or stent thrombosis [5]. |
|---|---|
| Related Catalog | |
| References |
[1]. Schr?r K. The pharmacology of cilostazol. Diabetes Obes Metab. 2002 Mar;4 Suppl 2:S14-9. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 664.7±55.0 °C at 760 mmHg |
| Melting Point | 159-160ºC |
| Molecular Formula | C20H27N5O2 |
| Molecular Weight | 369.461 |
| Flash Point | 355.8±31.5 °C |
| Exact Mass | 369.216461 |
| PSA | 81.93000 |
| LogP | 3.05 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.676 |
| Storage condition | Store at RT |
| Water Solubility | DMSO: 18 mg/mL, soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | VC8277500 |
| HS Code | 2933990090 |
|
~88% 
Cilostazol CAS#:73963-72-1 |
| Literature: OTSUKA PHARMACEUTICAL CO., LTD. Patent: EP1489080 A1, 2004 ; Location in patent: Page 6 ; |
|
~90% 
Cilostazol CAS#:73963-72-1
Detail
|
| Literature: YUHAN CORPORATION Patent: WO2006/22488 A1, 2006 ; Location in patent: Page/Page column 3 ; |
|
~99% 
Cilostazol CAS#:73963-72-1 |
| Literature: YUHAN CORPORATION Patent: WO2006/22488 A1, 2006 ; Location in patent: Page/Page column 3-4 ; |
|
~98% 
Cilostazol CAS#:73963-72-1 |
| Literature: YUHAN CORPORATION Patent: WO2006/22488 A1, 2006 ; Location in patent: Page/Page column 4; 5; 6 ; |
|
~97% 
Cilostazol CAS#:73963-72-1 |
| Literature: YUHAN CORPORATION Patent: WO2006/22488 A1, 2006 ; Location in patent: Page/Page column 3 ; |
|
~40% 
Cilostazol CAS#:73963-72-1 |
| Literature: Naddaka, Vladimir; Davidi, Guy; Saeed, Shady; Arad, Oded; Kaspi, Joseph Patent: US2005/222202 A1, 2005 ; Location in patent: Page/Page column 5; 9 ; |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Stimulation of angiogenesis by cilostazol accelerates fracture healing in mice.
J. Orthop. Res. 33 , 1880-7, (2015) Cilostazol, a selective phosphodiesterase-3 inhibitor, is known to control cyclic adenosine monophosphate (c-AMP) and to stimulate angiogenesis through upregulation of pro-angiogenic factors. There is... |
|
|
Comparative effects of the anti-platelet drugs, clopidogrel, ticlopidine, and cilostazol on aspirin-induced gastric bleeding and damage in rats.
Life Sci. 110(2) , 77-85, (2014) The present study compared the effects of frequently used anti-platelet drugs, such as clopidogrel, ticlopidine, and cilostazol, on the gastric bleeding and ulcerogenic responses induced by intralumin... |
|
|
Practical method for preparing nanosuspension formulations for toxicology studies in the discovery stage: formulation optimization and in vitro/in vivo evaluation of nanosized poorly water-soluble compounds.
Chem. Pharm. Bull. 62(11) , 1073-82, (2014) The present study aimed to develop a practical method for preparing nanosuspension formulations of poorly water-soluble compounds for enhancing oral absorption in toxicology studies in the discovery s... |
| 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydrochinolin-2(1H)-on |
| RETAL |
| Cilostazol |
| 2(1H)-Quinolinone, 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro- |
| Cilostal |
| CILOSTAZOLE |
| Pletal |
| MFCD00866780 |
| 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-2(1H)-quinolinone |
| PLETAAL |
| CILASTAZOL |
| 6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydroquinolin-2(1H)-one |
| CILOSTAZOL JP |
![6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)-butoxy]-1-[4-(1-cyclohexyl-1H-tetrazol-5-yl)-butyl]-3,4-dihydro-1H-quinolin-2-one structure](https://image.chemsrc.com/caspic/489/865792-18-3.png)
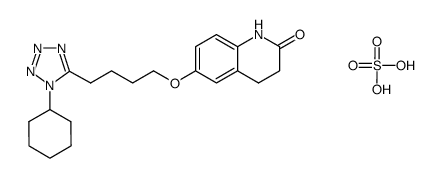

 CAS#:87153-03-5
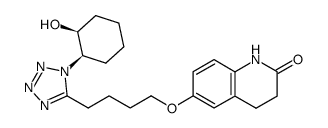
CAS#:87153-03-5![3,4-DIHYDRO-6-[4-[1-(TRANS-4-HYDROXYCYCLOHEXYL)-1H-TETRAZOL-5-YL]BUTOXY]-2(1H)-QUINOLINONE structure](https://image.chemsrc.com/caspic/318/87153-04-6.png) CAS#:87153-04-6
CAS#:87153-04-6 CAS#:87153-06-8
CAS#:87153-06-8
