α-Carotene

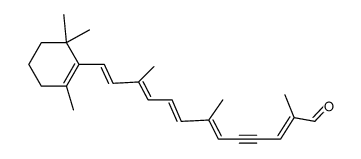
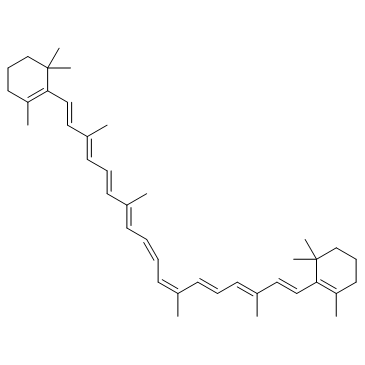
α-Carotene structure
|
Common Name | α-Carotene | ||
|---|---|---|---|---|
| CAS Number | 7488-99-5 | Molecular Weight | 536.873 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 644.9±35.0 °C at 760 mmHg | |
| Molecular Formula | C40H56 | Melting Point | 185ºC | |
| MSDS | USA | Flash Point | 341.2±20.8 °C | |
Use of α-Caroteneα-Carotene, a precursor of vitamin A, is used as an anti-metastatic agent or as an adjuvant for anti-cancer drugs. α-Carotene is isolated from yellow-orange and dark-green vegetables[1][2]. |
| Name | (6'R)-β,ε-carotene |
|---|---|
| Synonym | More Synonyms |
| Description | α-Carotene, a precursor of vitamin A, is used as an anti-metastatic agent or as an adjuvant for anti-cancer drugs. α-Carotene is isolated from yellow-orange and dark-green vegetables[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | α-Carotene (0.5-2.5 μM; 24 hours) significantly increases protein expression of TIMP-1 and TIMP-2 in a concentration-dependent manner in LLC cells. AC (0.5-2.5 μM) significantly increases PAI-1 protein expression. α-Carotene (2.5 μM) also significantly inhibits integrin β1-mediated phosphorylation of focal adhesion kinase (FAK) which then decreased the phosphorylation of MAPK family[2]. α-Carotene (0.5, 1, 2.5 μM; 48 hours) significantly and concentration-dependently inhibits invasion of LLC during 48 h of incubation[2]. α-Carotene (0.5, 1, 2.5 μM; 24 hours) significantly decreases activities of MMP-9, -2 and uPA in concentration-dependent manner in LLC cells[2]. α-Carotene (2, 5, 10 μM; 7 days) inhibits the proliferation of the human neuroblastoma cell line GOTO in a dose- and time-dependent manner. α-Carotene (5 μM; 48 hours) halts the cell cycle at the G0/G1 phase concomitantly with a reduction in the mRNA expression of the protooncogene N-Myc[3]. Western Blot Analysis[2] Cell Line: Lewis lung carcinoma (LLC) cells Concentration: 0.5, 1, 2.5 μM Incubation Time: 24 hours Result: Significantly increased protein expression of TIMP-1 and TIMP-2 in a concentration-dependent manner in LLC cells. |
| In Vivo | α-Carotene (5 mg/kg; oral; twice a week; for additional 3 weeks) alone significantly decreases lung metastasis without affecting primary tumor growth[2]. Animal Model: C57BL/6 male mice (4 weeks old; 20-25 g) with LLC cells[2] Dosage: 5 mg/kg Administration: Oral; twice a week; for additional 3 weeks Result: Significantly decreased lung metastasis. |
| References |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 644.9±35.0 °C at 760 mmHg |
| Melting Point | 185ºC |
| Molecular Formula | C40H56 |
| Molecular Weight | 536.873 |
| Flash Point | 341.2±20.8 °C |
| Exact Mass | 536.438232 |
| LogP | 15.45 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.563 |
| Storage condition | -20°C |
|
~% 
α-Carotene CAS#:7488-99-5 |
| Literature: Phytochemistry (Elsevier), , vol. 6, p. 1119 - 1126 |
|
~% 
α-Carotene CAS#:7488-99-5 |
| Literature: Phytochemistry (Elsevier), , vol. 6, p. 1119 - 1126 |
|
~% 
α-Carotene CAS#:7488-99-5 |
| Literature: Helvetica Chimica Acta, , vol. 38, p. 610 |
|
~% 
α-Carotene CAS#:7488-99-5 |
| Literature: Helvetica Chimica Acta, , vol. 44, p. 985 - 993 |
|
~% 
α-Carotene CAS#:7488-99-5 |
| Literature: Helvetica Chimica Acta, , vol. 44, p. 985 - 993 |
|
~% 
α-Carotene CAS#:7488-99-5 |
| Literature: Helvetica Chimica Acta, , vol. 44, p. 985 - 993 |
|
~% 
α-Carotene CAS#:7488-99-5 |
| Literature: Helvetica Chimica Acta, , vol. 44, p. 985 - 993 |
|
~% 
α-Carotene CAS#:7488-99-5 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 588, p. 117,123 |
|
~% 
α-Carotene CAS#:7488-99-5 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 588, p. 117,123 |
|
A dietary guideline adherence score is positively associated with dietary biomarkers but not lipid profile in healthy children.
J. Nutr. 145(1) , 128-33, (2015) Whether dietary indexes are associated with biomarkers of children's dietary intake is unclear.The study aim was to examine the relations between diet quality and selected plasma biomarkers of dietary... |
|
|
Avocado consumption enhances human postprandial provitamin A absorption and conversion from a novel high-β-carotene tomato sauce and from carrots.
J. Nutr. 144(8) , 1158-66, (2014) Dietary lipids have been shown to increase bioavailability of provitamin A carotenoids from a single meal, but the effects of dietary lipids on conversion to vitamin A during absorption are essentiall... |
|
|
Skin carotenoids: a biomarker of fruit and vegetable intake in children.
J. Acad. Nutr. Diet. 114(8) , 1174-80, (2014) Studies of adult subjects have found a strong correlation between serum carotenoids and skin carotenoids measured by resonance Raman spectroscopy (RRS). No published studies have examined correlations... |
| 1,3,3-Triméthyl-2-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tétraméthyl-18-(2,6,6-triméthyl-2-cyclohexén-1-yl)-1,3,5,7,9,11,13,15,17-octadécanonaèn-1-yl]cyclohexène |
| CISALPHA-CAROTENE |
| Carotene |
| 1,3,3-Trimethyl-2-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethyl-2-cyclohexen-1-yl)-1,3,5,7,9,11,13,15,17-octadecanonaen-1-yl]cyclohexen |
| Renieratene |
| (6'R)-beta,epsilon-carotene |
| Isorenieratene |
| A-CAROTENE |
| MFCD00136012 |
| CAROTENE,A |
| α-Carotene |
| 1,3,3-Trimethyl-2-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-3,7,12,16-tetramethyl-18-(2,6,6-trimethyl-2-cyclohexen-1-yl)-1,3,5,7,9,11,13,15,17-octadecanonaen-1-yl]cyclohexene |
| all-trans-α-carotene |
| β,β-Carotene, 4,5-didehydro-5,6-dihydro- |
| 4,5-Didehydro-5,6-dihydro-β,β-carotene |
| (+)-α-Carotene |
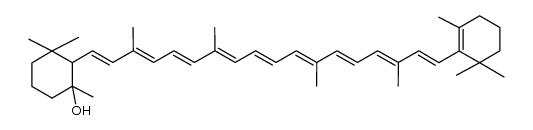


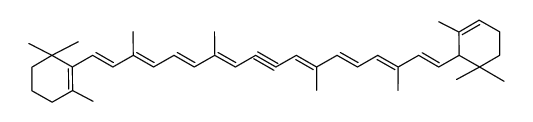

 CAS#:7235-40-7
CAS#:7235-40-7
