Boc-Cys(NPys)-OH
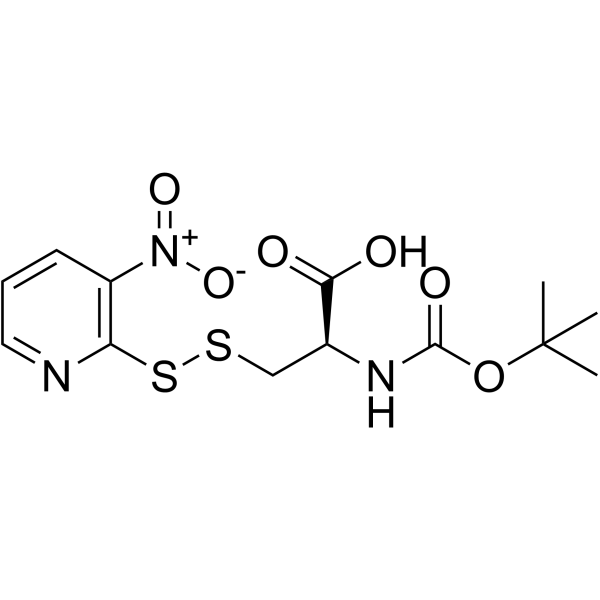
Boc-Cys(NPys)-OH structure
|
Common Name | Boc-Cys(NPys)-OH | ||
|---|---|---|---|---|
| CAS Number | 76880-29-0 | Molecular Weight | 375.421 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 553.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C13H17N3O6S2 | Melting Point | -160ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | 288.8±30.1 °C | |
Use of Boc-Cys(NPys)-OHBoc-Cys(Npys)-OH is a cysteine derivative[1]. |
| Name | (2R)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-3-[(3-nitropyridin-2-yl)disulfanyl]propanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Boc-Cys(Npys)-OH is a cysteine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 553.9±50.0 °C at 760 mmHg |
| Melting Point | -160ºC (dec.) |
| Molecular Formula | C13H17N3O6S2 |
| Molecular Weight | 375.421 |
| Flash Point | 288.8±30.1 °C |
| Exact Mass | 375.055878 |
| PSA | 184.94000 |
| LogP | 4.25 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.616 |
|
Material Safety Data Sheet
Section1. Identification of the substance Product Name: Boc-cys(npys)-oh Synonyms: Section2. Hazards identification Harmful by inhalation, in contact with skin, and if swallowed. Section3. Composition/information on ingredients. Ingredient name:Boc-cys(npys)-oh CAS number:76880-29-0 Section4. First aid measures Skin contact:Immediately wash skin with copious amounts of water for at least 15 minutes while removing contaminated clothing and shoes. If irritation persists, seek medical attention. Eye contact:Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical attention. Inhalation:Remove to fresh air. In severe cases or if symptoms persist, seek medical attention. Ingestion:Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention. Section5. Fire fighting measures In the event of a fire involving this material, alone or in combination with other materials, use dry powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus should be worn. Section6. Accidental release measures Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national standards. Respiratory precaution:Wear approved mask/respirator Hand precaution:Wear suitable gloves/gauntlets Skin protection:Wear suitable protective clothing Eye protection:Wear suitable eye protection Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container for disposal. See section 12. Environmental precautions: Do not allow material to enter drains or water courses. Section7. Handling and storage Handling:This product should be handled only by, or under the close supervision of, those properly qualified in the handling and use of potentially hazardous chemicals, who should take into account the fire, health and chemical hazard data given on this sheet. Store in closed vessels. Storage: Section8. Exposure Controls / Personal protection Engineering Controls: Use only in a chemical fume hood. Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles. General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse. Section9. Physical and chemical properties Appearance:Not specified Boiling point:No data No data Melting point: Flash point:No data Density:No data Molecular formula:C13H17N3O6S2 Molecular weight:375.4 Section10. Stability and reactivity Conditions to avoid: Heat, flames and sparks. Materials to avoid: Oxidizing agents. Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, sulfur oxides. Section11. Toxicological information No data. Section12. Ecological information No data. Section13. Disposal consideration Arrange disposal as special waste, by licensed disposal company, in consultation with local waste disposal authority, in accordance with national and regional regulations. Section14. Transportation information Non-harzardous for air and ground transportation. Section15. Regulatory information No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302, or have known CAS numbers that exceed the threshold reporting levels established by SARA Title III, Section 313. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
|
~90% 
Boc-Cys(NPys)-OH CAS#:76880-29-0 |
| Literature: Matsueda, Rei; Higashida, Susumu; Ridge, Richard J.; Matsueda, Gary R. Chemistry Letters, 1982 , p. 921 - 924 |
|
~87% 
Boc-Cys(NPys)-OH CAS#:76880-29-0 |
| Literature: Matsueda, Rei; Higashida, Susumu; Ridge, Richard J.; Matsueda, Gary R. Chemistry Letters, 1982 , p. 921 - 924 |
|
~90% 
Boc-Cys(NPys)-OH CAS#:76880-29-0 |
| Literature: Matsueda, Rei; Higashida, Susumu; Ridge, Richard J.; Matsueda, Gary R. Chemistry Letters, 1982 , p. 921 - 924 |
|
~64% 
Boc-Cys(NPys)-OH CAS#:76880-29-0 |
| Literature: Matsueda, Rei; Higashida, Susumu; Ridge, Richard J.; Matsueda, Gary R. Chemistry Letters, 1982 , p. 921 - 924 |
|
~% 
Boc-Cys(NPys)-OH CAS#:76880-29-0 |
| Literature: Synthesis, , # 1 p. 21 - 22 |
|
~% 
Boc-Cys(NPys)-OH CAS#:76880-29-0 |
| Literature: Synthesis, , # 1 p. 21 - 22 |
|
~% 
Boc-Cys(NPys)-OH CAS#:76880-29-0 |
| Literature: Synthesis, , # 1 p. 21 - 22 |
| Precursor 8 | |
|---|---|
| DownStream 3 | |
|
Controlled peptide-protein conjugation by means of 3-nitro-2-pyridinesulfenyl protection-activation.
Int. J. Pept. Protein Res. 32(3) , 161-6, (1988) The disulfide bond in S-3-nitro-2-pyridinesulfenyl (S-Npys) compounds is stable towards the acid treatment used in solid-phase peptide synthesis, yet the liability of S-Npys-peptides towards nucleophi... |
|
|
Preparation of Boc-[S-(3-nitro-2-pyridinesulfenyl)]-cysteine and its use for unsymmetrical disulfide bond formation.
Int. J. Pept. Protein Res. 28(2) , 107-12, (1986) The 3-nitro-2-pyridinesulfenyl (Npys) derivative of cysteine was prepared and used to facilitate the formation of an unsymmetrical disulfide bond. Since this derivative is stable in trifluoroacetic ac... |
|
|
Effects of sulfhydryl-modifying reagents, 3-nitro-2-pyridinesulfenyl compounds, on the coupling between inhibitory receptors and GTP-binding proteins Gi/Go in rat brain membranes.
Mol. Pharmacol. 38(2) , 184-91, (1990) To gain insight into the coupling mechanism of inhibitory receptors, 5-hydroxytryptamine1A receptors and alpha 2-adrenoceptors, with GTP-binding proteins (G proteins) in the central nervous system, we... |
| N-{[(2-Methyl-2-propanyl)oxy]carbonyl}-3-[(3-nitro-2-pyridinyl)disulfanyl]-L-alanine |
| n-(tert-butoxycarbonyl)-3-[(3-nitropyridin-2-yl)disulfanyl]-l-alanine |
| Boc-Cys-S-(3-nitro-2-pyridinesulfenyl) |
| Boc-cys(npys) |
| N-t-butoxycarbonyl-S-(3-nitro-2-pyridylthio)-L-cysteine |
| t-Bcnp |
| Boc-cys(npys)-OH |
| MFCD00153301 |
| L-Alanine, N-[(1,1-dimethylethoxy)carbonyl]-3-[(3-nitro-2-pyridinyl)dithio]- |
| Nalpha-Boc-S-(3-nitro-2-pyridylthio)-L-cysteine |

![2-[(4-methoxyphenyl)methylsulfanyl]-3-nitropyridine structure](https://image.chemsrc.com/caspic/413/153815-22-6.png)
 CAS#:38240-29-8
CAS#:38240-29-8 CAS#:20887-95-0
CAS#:20887-95-0 CAS#:10389-65-8
CAS#:10389-65-8
